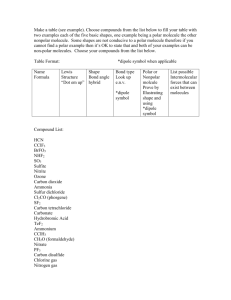
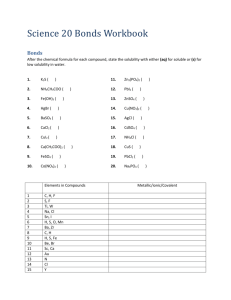
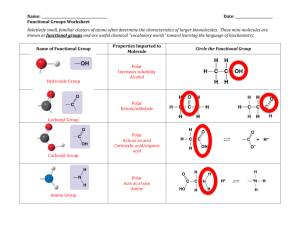
Ut *,->•£• _A
advertisement
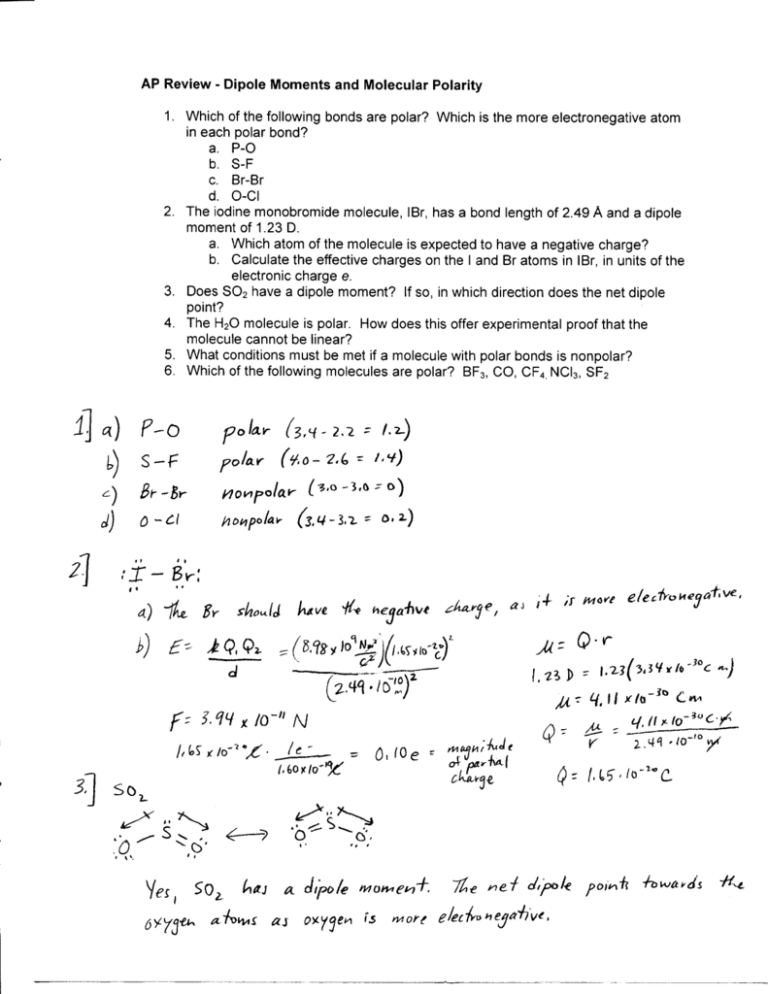
AP Review - Dipole Moments and Molecular Polarity 1. Which of the following bonds are polar? Which is the more electronegative atom in each polar bond? a. P-O b. S-F c. Br-Br d. O-CI 2. The iodine monobromide molecule, IBr, has a bond length of 2.49 A and a dipole moment of 1.23 D. a. Which atom of the molecule is expected to have a negative charge? b. Calculate the effective charges on the I and Br atoms in IBr, in units of the electronic charge e. 3. Does SO2 have a dipole moment? If so, in which direction does the net dipole point? 4. The HaO molecule is polar. How does this offer experimental proof that the molecule cannot be linear? 5. What conditions must be met if a molecule with polar bonds is nonpolar? 6. Which of the following molecules are polar? BF3, CO, CF4, NCI3, SF2 P-O A Bl"& /0~" N . \,Ut *,->•£• _A— = 0 , ' O e ' ?n 1J so t '-""' ,. .0 aj '"5 more e'leek* »e '# a p olar p C.J u