HC Assignment: Table of Molecules depicting each shape, polar
advertisement
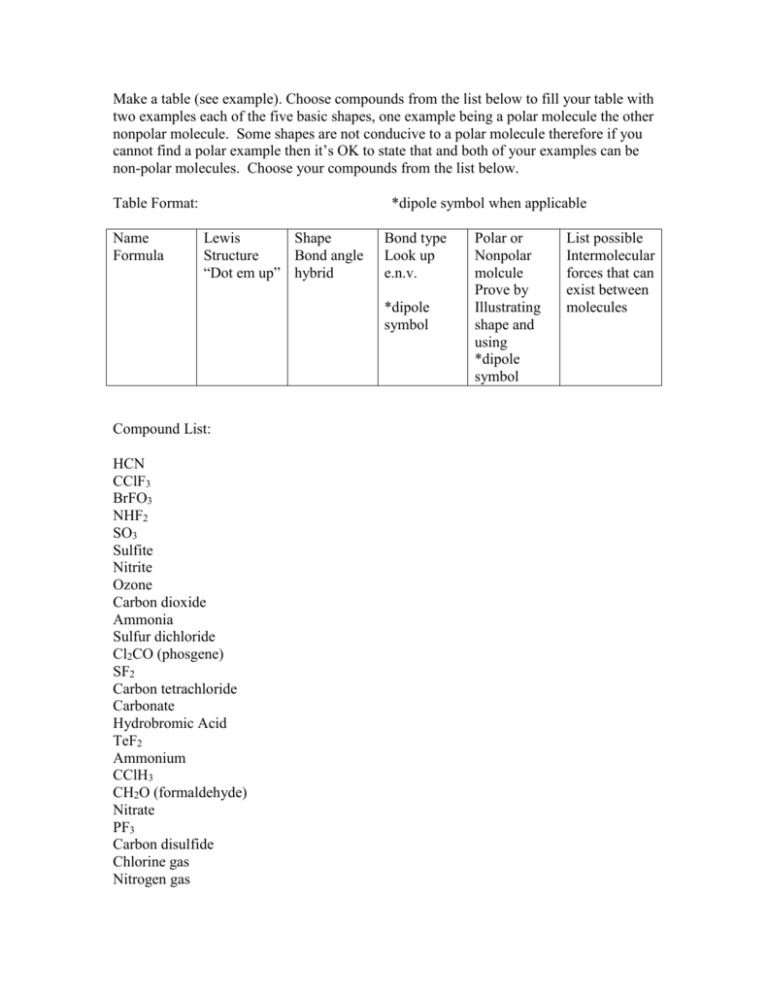
Make a table (see example). Choose compounds from the list below to fill your table with two examples each of the five basic shapes, one example being a polar molecule the other nonpolar molecule. Some shapes are not conducive to a polar molecule therefore if you cannot find a polar example then it’s OK to state that and both of your examples can be non-polar molecules. Choose your compounds from the list below. Table Format: Name Formula *dipole symbol when applicable Lewis Shape Structure Bond angle “Dot em up” hybrid Bond type Look up e.n.v. *dipole symbol Compound List: HCN CClF3 BrFO3 NHF2 SO3 Sulfite Nitrite Ozone Carbon dioxide Ammonia Sulfur dichloride Cl2CO (phosgene) SF2 Carbon tetrachloride Carbonate Hydrobromic Acid TeF2 Ammonium CClH3 CH2O (formaldehyde) Nitrate PF3 Carbon disulfide Chlorine gas Nitrogen gas Polar or Nonpolar molcule Prove by Illustrating shape and using *dipole symbol List possible Intermolecular forces that can exist between molecules Water











