Electrolytes vs Non-electrolytes: Chemistry Notes
advertisement
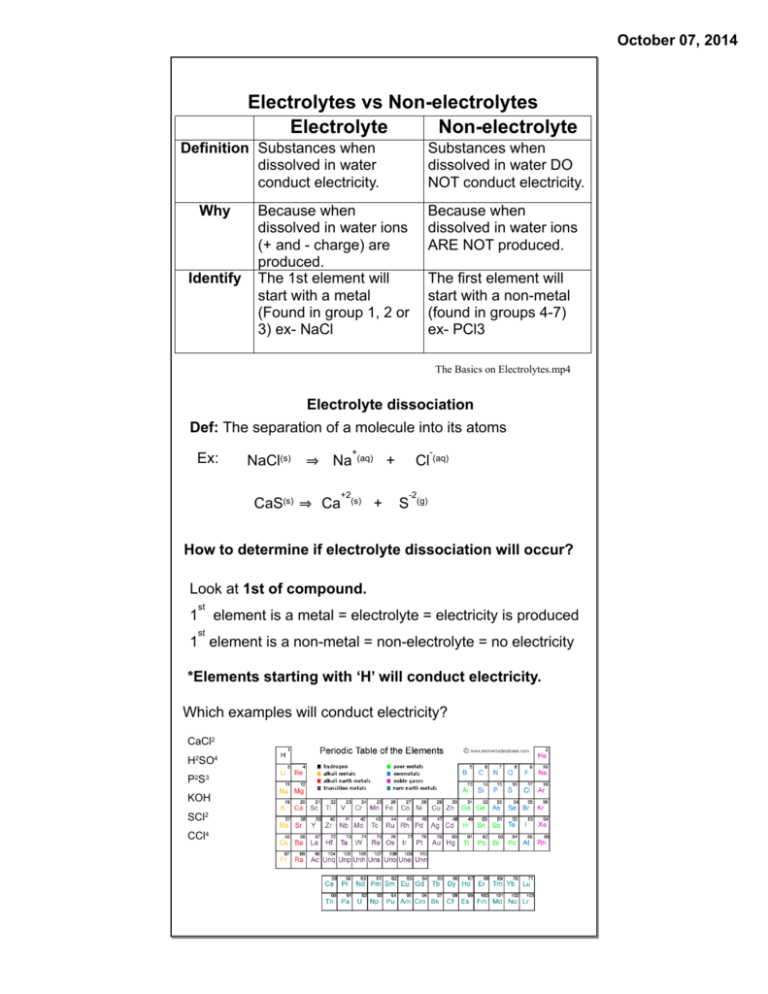
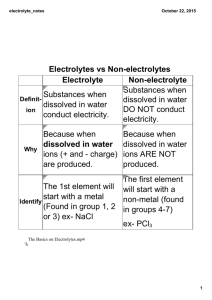
October 07, 2014 Electrolytes vs Non-electrolytes Electrolyte Non-electrolyte Definition Substances when dissolved in water conduct electricity. Why Identify Substances when dissolved in water DO NOT conduct electricity. Because when dissolved in water ions (+ and - charge) are produced. The 1st element will start with a metal (Found in group 1, 2 or 3) ex- NaCl Because when dissolved in water ions ARE NOT produced. The first element will start with a non-metal (found in groups 4-7) ex- PCl3 The Basics on Electrolytes.mp4 Electrolyte dissociation Def: The separation of a molecule into its atoms Ex: NaCl(s) CaS(s) Na Ca +2 + (aq) (s) - + + Cl (aq) S -2 (g) How to determine if electrolyte dissociation will occur? Look at 1st of compound. 1 st element is a metal = electrolyte = electricity is produced st 1 element is a non-metal = non-electrolyte = no electricity *Elements starting with ‘H’ will conduct electricity. Which examples will conduct electricity? CaCl2 H2SO4 P2S3 KOH SCl2 CCl4 October 07, 2014 Conduction capabilities October 07, 2014 Types of electrolytes Base Acid Definition · · · pH less than 7 · · · pH greater · · · made of than 7 metals bonded · · · taste sour to non-metals · · · turn blue litmus · · · bitter, (KCl, CaCl2, slippery paper RED MgBr2) · · · turn red · · · when dissolved, · · · have no litmus paper separate into H+ effect on litmus and a negative ion BLUE paper · · · when · · · (diagram) · · · important in dissolved, · · · start with human body separate into "H" (HCl, H2SO4) (minerals), soil, OH- and fertilizer positive ion (diagram) · · · end in "OH" (NaOH, Mg(OH)2 negative ion · · · OH- and positive ion · · · made of metals bonded to non-metals BRA (Blue -> Red = Acid) RBB (Red -> Blue = Base) have no effect on litmus paper Electrolyte · · · H+ and a Litmus paper Salt Found in Fruits, cleaning products, batteries Recognize H Examples Blood, cleaning Foods, human products body, soil OH NaOH NaCl, KCl, HCl Exceptions H2O CH3COOH From the molecular formula, how can you determine if a substance is a nonelectrolyte? October 07, 2014 False acids and bases (organic molecules) · molecules that may start with H or end in OH but are NOT electrolytes · these molecules contain carbon (C) · they do not separate into ions, therefore do not conduct electricity · they are neutral Ex. CH3 OH, C2 H5 OH, CH3 COOH October 07, 2014 October 07, 2014 October 07, 2014