Solute vs. Solvent
advertisement

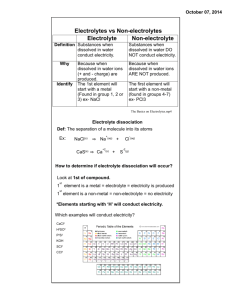
Chapter 5 Solutions! What is a Solution? Fill in Table on Pg. 188 #1: A solution is a homogenous mixture of substances composed of at least one solute dissolved in a solvent. Characteristics of solutions: -Particles cannot be seen with the naked eye -Particles cannot be separated by filtering -Solutions are transparent Solute vs. Solvent Solute – The substance that is dissolved (e.g. NaCl) Solvent – The substance that the solute is dissolved in (e.g. Water) Solutes and solvents may be all three phases: Table 1 – pg. 192: Solute in solvent Example solution Source Gas in gas Gas in liquid Gas in solid Liquid in liquid Solid in liquid Solid in solid Oxygen in Nitrogen Oxygen in water Oxygen in ice Methanol in water Sugar in water Tin in Copper Air Water (ocean) Ice Antifreeze Syrup Bronze alloy Steel is a solution (alloy) of iron in carbon, stainless steel also contains chromium. In this chapter we will focus on solutions that use water as their solvent. Aqueous Solutions If a solution uses water as a solvent, we call it an aqueous solution. We can indicate this in a chemical equation by adding (aq). NH3(aq) – Ammonia gas (solute) dissolved in water (solvent) Other solvents can be shown in the same way, but for this chapter we will deal primarily with aqueous solutions. Water is a very important part of life. Many chemical reactions require water to occur, even though the water itself may not react. Acids & Bases Acids -have pH < 7 -turn litmus red -ionize in water to produce H+ ions -conduct electricity when in a solution -taste sour Bases -have pH > 7 -turn litmus blue -dissociate in water to produce OH- ions -conduct electricity when in a solution -taste bitter -feel slippery Examples: Examples: Hydrochloric acid - HCl(aq) Acetic acid – CH3COOH(aq) Sodium hydroxide - NaOH(aq) Potassium hydroxide – KOH(aq) Electrolytes vs. Non-electrolytes An electrolyte is a solution that conducts electricity; these are mostly highly soluble ionic compounds, including acids and bases. Molecular compounds do not usually conduct electricity, therefore they are non-electrolytes. *It is important to note that an ionic compound by itself will not conduct electricity, only when it is in a solution! (or melted)* Video: Conductivity of compounds http://www.youtube.com/watch?v=4FLFy3mrjD4 Video: Conductivity of molten sodium chloride http://www.youtube.com/watch?v=NfNIn4R8tg4 Common Properties (Review) Ionic compounds - Metals give up electrons to non metals - When soluble they dissociate in water into IONS - Solutions are (mostly) neutral in pH and conduct electricity Molecular Compounds - Share electrons (nonmetal and nonmetal) - When soluble they separate in water to form individual MOLECULES - Solutions are neutral in pH and do not conduct electricity Strong Acids - turn litmus paper red - ionizes to produce hydrogen ions H+ in water (not ionic compounds though) - are electrolytes when dissolved in water Bases - turns litmus paper blue - dissociates to produce hydroxide (OH-) ions in water (they’re ionic) - are electrolytes when in aqueous solutions [Do Lab Exercise 5.A on page 195] 5.1 Chemical Analysis pg. 194 or pg. 227 *must be done for the start of lab Problem: copy from text Prediction: Substance Solubility in Water Litmus Conductivity Design: Summarize lab in 2 –3 sentences. M.V. & R.V Procedure: very detailed, anyone should be able to follow Evidence: setup a table to record data Substance Solubility Litmus Water 1 2 3 4 Conductivity Analysis: Identify 4 unknowns (include a brief explanation for each) Evaluation : - comment of lab (is procedure adequate at finding unknown solids?) H.W. 1) Lab Exercise 5-A pg. 195 2) pg. 195 #2, 4,5,6 3) prelab