Escobedo Electrolyte Challenge Cristian Escobedo January 31
advertisement

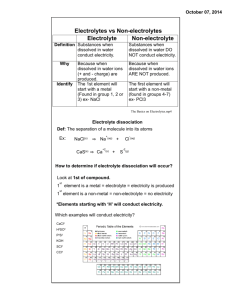
Electrolyte Challenge Cristian Escobedo January 31, 2012 7th Escobedo 2 Table of contents A. Abstract B. Question C. Hypothesis D. Research E. Materials F. Procedure G. Data H. Conclusion I. Acknowledgement J. Works Cited Escobedo 3 A. Abstract What is the function of electrolytes in our bodies? Electrolytes are used for energy. All living organisms need electrolytes. This experiment requires testing electrolytes in orange juice and sports drinks. I will use a plastic tube, which will be wrapped with a wire and then put the tube with the wire in the sports drink and orange juice. Lastly, I used the meter to measure electrolytes in each liquid used for the experiment. After the experiment was completed I determined that my initial hypothesis was incorrect. Escobedo 4 B. Questions What are the amounts of sodium, potassium, and carbohydrates in one serving of orange juice? How does this compare with sports drinks? What do electrolytes do in your body? C. Hypothesis I think that the sports drinks will not have a lot of electrolytes and I think that the juices will have a lot of electrolytes in it because of the protein and vitamins. Escobedo 5 D. Research Benjamin Franklin was the first person the find electricity. He said that lightning was a huge electric spark. He made a kite and he out a key on the kite and then one day a great storm with strong winds. The lightning touched the key the electricity went to the key and when he touch the key a spark jumped across and touch his finger. People have grown orange more for 4000 years. In A.D. 800s Arab traded oranges into Africa. In the 1400s Portuguese traveled with oranges from India to Europe. In the 1500s the Europeans planted oranges in Florida and Brazil. The Italian explorer Christopher Columbus brought orange seeds in the 1493. Thomas Edison made the first meter in 1882. “The current passed through copper sulfate solution.” (pgs. 46-47 by Steve Parkers) In the 1930s they made a spinning plate. Electrolytes are in drinks they make you gain energy. Ammeter shaft is suspended between North and South permanent magnet. Ammeter measure current range from a few millionths. Alternating current means that the circuit is a type of AC. The AC uses energy from the power plants. A multimeter measures electrical properties like a DC voltage is current and resistance. Meters and a multimeter combine voltmeter and an ammeter. Electricians use multimeter on batteries like switches, motors. A voltmeter and a multimeter measure the amount of AC or DC voltage circuit. Electricity is a basic feature of mater that comes from the universe. Electricity makes all powered devices like IPods, lights, TVs and all devices. Every device is made up of electricity. Escobedo 6 Everything is made up of electricity. ‘’Electricity makes these and many other useful things possible.’’(pg. 190 world book 2002) Electricity and magnetism make a force called electromagnetism. We use electricity in our homes like dishwashers and televisions. Electricity is in batteries and batteries have electrolytes in them batteries. Electrolytes are batteries that can be liquid and the liquid is the electricity. Ammeter is a meter that measures circuit. It can find volts and Ohms to help people to find ohms and volts. It is like a switch to find volts. Ion is atoms or a group of atoms has electric charge. Atoms become charged and gains electrons and lose electrons. An atom has negatively around small, heavy nucleus. Volts are in light bulb and volts are electricity that turns the light on and off. Most batteries and other sources use force. Open circuit is turn on switch. That lets the electricity go in to anything. Each electron has a negative charge and the nucleus balances the positive charge. The electric current occurs moves the electrons to its place. Free electrons are to produce an electric current. An electron removes positive atoms and changes it to negative ions. Electrons gain or lose atoms or molecules. A flow of electric charge is called electric current. Energy is the flow of current. A current flow thought electric devices, energy will convert into current. Electricity is a form of energy with atoms is called electrons it has a negative charge and a positive charge. Alessandro Volta, an Italian professor, devised the first battery in 1800. One if Escobedo 7 the very basic from of the cell is called a simple voltaic cell. When cell are in use the molecules of the acid in the electrolytes. Electrically charged portions called ions. Electrolytes basically exist as acids, bases or salts within the body. The main types of electrolytes in your body are sodium, potassium, calcium, magnesium, chloride, phosphate and bicarbonate. In order to function properly, the body must contain a proper electrolyte balance inside and outside of your cells, so they can properly transport water to and from the body’s major systems. This essential electrolytes balance is kept in check by eating and drinking things that contain electrolytes. Electricity is in our body it is the one that keep us alive. An electrolyte is a "medical/scientific" term for salts, specifically ions. The term electrolyte means that this ion is electrically-charged and moves to either a negative or positive electrode. Electrolytes are important because they are what your cells use to maintain voltages across their cell membranes and to carry electrical impulses across themselves and to other cells. An electrolyte is "any compound that, in solution or in molten form, conducts electricity and is decomposed by it. It is an ionizable substance in solution. Liquid electrolyte solution plays a key role in transporting the positive Lithium ions between the cathode and anode. Electrolytes are good for people because it makes us gain more energy in our body it makes us run more and if you lose energy with the electrolytes you will gain more and keep running. Many sports drinks contain added potassium and sodium to help restore the body's proper electrolyte balance after intense physical exertion. Escobedo 8 E. Material I will be using copper wire, bare, 24-gauge,wire cutters, Ruler, plastic tube from a pen, cut to about 1 inch, or small plastic toys in the shape of a tube and 1 inch long, Handsaw to cut plastic tube, 9-V battery, 9-V battery clip, wires with alligator clips for making connections, should have clips on both ends, and be about 6 inches long, small bowls plastic, glass, or ceramic bowls, not metal, masking tape, permanent marker, sports drink of your choice, room-temperature, orange juice of your choice, room-temperature, distilled water (dH2O), room-temperature, Tap water, room-temperature, measuring cup, 1/2-cup capacity, A digital multimeter. The multimeter has various sensitivity settings. Some multimeters automatically change sensitivity, but most inexpensive ones have a dial that sets the sensitivity. Typical multimeters have a range of settings from 200 microamps up to 200 milliamps. This is a thousand-fold range, The 200microamp setting is good for measuring from 0–200 microamps, but will not work for higher currents, 200-milliamp setting is good for 20–200 milliamp currents, but is inaccurate for low currents, paper towels, lab notebook. Escobedo 9 F. Procedure Using the wire cutters cut two pieces of copper wire, each about 6 inches long. Using your handsaw, cut the plastic tube into a 1-inch piece. Ask an adult for assistance with this step. Wrap one piece of the wire around the tube near one end a few times, leaving about 2 inches of wire free. Wrap the other piece of wire around the tube at the other end a few times, again, leaving about 2 inches of wire free. There should be no contact between the two wires, and they should be wrapped tight enough that they won't slide off of the tube. See Figure 1 for a visual aid. The conductance sensor consists of a non-conducting core (plastic or rubber) with copper wire wrapped around the ends. The ions in the solution complete the circuit and allow current to flow between the copper wires. Attach the battery clip onto the battery. Attach one of the free copper wires on the conductance sensor to the positive terminal of the 9-V battery, using the wire with one of the alligator clips. Attach the other copper wire from the sensor to the black terminal of the multimeter, using the other alligator clip. It does not matter which side has the positive and negative leads. Note that this is an open circuit because of the gap between the wires wrapped around the non-conducting tube. You will use the electrolytes in the solutions to close the circuit. The amount of current that flows is proportional to the electrolyte concentration. Escobedo 10 G. Data 8.2 8.1 8 Quench 7.9 Powerade 7.8 Orange juice Water 7.7 7.6 7.5 F irst time Second time Third time Escobedo 11 H. Conclusion My hypothesis was incorrect because the Powerade had more electrolytes and I thought that the orange juice would have more because it is a natural fruit juice. I. Acknowledgement I thank mom and my dad for buying the things for me and I thank my brother and I thank God for helping me to pick my project and making me think. Escobedo 12 Works Cited Freeman, Mae Blacker, Ira Maximilian Freeman, and René Martin. The Story of Electricity. New York: Random House, 1961. Print. Hartmut, Walter S. "Oranges." The World Book. 1983. Print. Parker, Steve. Eyewitness Science: Electricity. New York: Dorling Kindersley, 1992. Print. Prograde Nutrition. Web. 24 Jan. 2012. <http://www.getprograde.com>. Rock, Peter A. "Multimeter." The World Book. 2002. Print. Web. 24 Jan. 2012. <http://www.ndt-ed.org>. "What Is a Multimeter?" WiseGEEK: Clear Answers for Common Questions. Web. 24 Jan. 2012. <http://www.wisegeek.com/what-is-a-multimeter.htm>. Whitehead, E. R. "Ammeter." The World Book. Vol. 1. Chicago: Scott Fetzer, 1984. 409+. Print. Wolfson, Richard. "Electricity." The World Book. 2002. Print. Wolfson, Richard. "Electrons." The World Book. 1992. Web. Wolfson, Richard. "Volts." The World Book. 2002. Print. "Electrolyte." Electrolyte Definition. Ed. Random House. Dictionary.com, 2009. Web. 25 Jan. 2012. http://dictionary.reference.com/browse/electrolyte