Molecular Modeling Activity Overview: Molecules and polyatomic
advertisement

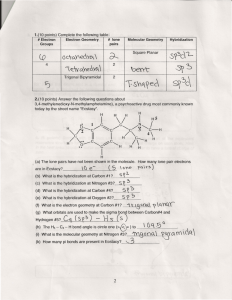
Molecular Modeling Activity Overview: Molecules and polyatomic ions are not all flat structures. Each has a three-dimensional shape that helps account for its various chemical and physical properties. This Activity aims to reinforce the conept of molecular geometry through the use of traditional molecular modeling kits, balloons, and computer generated images downloaded from the internet. I. Cooperative Learning Activity: Electron dot structures and structureal formulas usually show a given molecule in only two dimensions. In reality, many molecules and polyatomic ions exist in three dimensions. The VSEPR (valence shell electron-pair repulsion) theory provides an explanation for the shape of many molecules and ions. According to VSEPR theory, valence shell electron pairs will stay as far apart as possible so that the repulsions between them are minimized. Time Frame: Two 40 minute periods. Objectives: Define the VSEPR theory and explain its relationship to the shape of molecules. Differentiate electron pair geometry from molecular geometry. Name and describe the five electron pair geometries which can surround the central atom. Show how molecular geometry is a function of electron pair geometry. State the two factors that determine the polarity of a molecule. Explain how the geometry of a molecule helps to determine its properties. Organize your information in the form of a chart. Materials: Pens & pencils Textbook or other reference books Pear-shaped balloons (6 of one color, 2 of another) Molecule model building kit Instructions: 1. Build the following molecules using a molecule model building kit. Use wooden sticks for single bonds Use metal springs for multiple bonds NOTE: You may not be able to build ALL molecules with the kit. If not, rely on the balloon model. 2. Complete the table as instructed below. 3. Draw the Lewis-dot diagram for each molecule. 4. Sketch a 3-d diagram of each molecule. 5. Using balloons, show the geometry of the molecule. Use one color to represent bonding pairs. Use the other color to represent nonbonding pairs. 6. Identify the electron pair geometry by focusing on all the electron domains (ALL the balloons). 7. Identify the molecular geometry by focusing on only the bonded electron domains. 8. Complete the table, with the exception of “Polarity”. (We will address this later.) 9. Table 8.6 on page 394 of your Zumdahl, 6th ed. Chemistry textbook is helpful for determining shapes of molecules. NOTE: there is often a difference between the electron pair geometry and the molecular geometry. Electron pair geometry takes into account ALL of the electron domains. For example, NH3 has 4 electron domains, giving it a tetrahedral electron domain geometry. However, since only 3 of those domains have bonding electrons (atoms attached to them) the MOLECULAR geometry is pyramidal. Data Table: Draw table in notebook or continue on back of paper, if space is not sufficient. Molecule BeH2 Sketch of molecule Lewis dot diagram # of bonding pairs (edomains) # of nonbonding pairs (edomains) Electron pair geometry Molecular geometry Molecula r shape Polarity 2 0 Linear AX2 Linear nonpolar BH3 SO2 CH4 NH3 AX3E H2O PCl5 SF6 Molecular Geometry symbols: A= central atom X = non-central atoms E= non-bonding electron domains Drawing Sketches of Molecules: Dashed bonds are used to represent bonds that project backward (behind the drawing plane). Wedged bonds are used to represent bonds that project outward (in front of the drawing plane). The three common types of bonds used in drawing chemical structures: See CH4, above, for an example of how to represent the molecular shape.