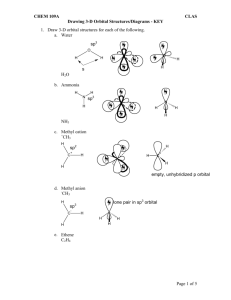
px pz H C N pz pz
advertisement

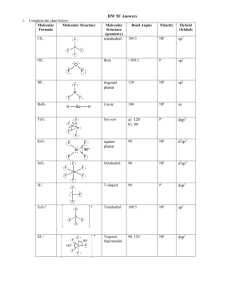
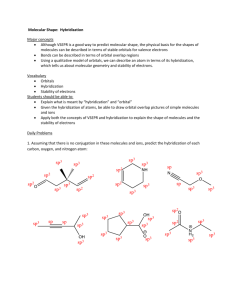
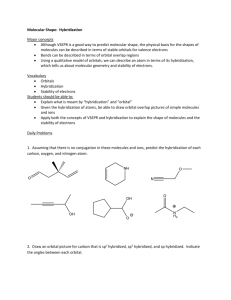
WKS Hybridization and Orbital diagrams 1) 2) 3) 4) 5) 6) 7) Name________________________________ Period_________ Date___________ Why are sp3, sp2 and sp orbitals all called hybrid orbitals? ___________________________________ How many sp3 orbitals does an sp3 hybridized atom have? What is the bond angle between them?___ How many sp2 orbitals does an sp2 hybridized atom have? ___What is the bond angle between them?___ How many sp orbitals does an sp hybridized atom have? ____What is the bond angle between them?____ If the overall shape is tetrahedral, there are (4, 3, 2) regions of e-s. Therefore, the orbitals would be (sp3, sp2 , sp) hybridized. If the overall shape is trigonal planar, there are (4, 3, 2) regions of e-s.Therefore, the orbitals would be (sp3, sp2, sp)hybridized. If the overall shape is linear, there are (4, 3, 2) regions of e-s. Therefore, the orbitals would be (sp3, sp2 , sp) hybridized. 8) Label the hybridization (sp3, sp2 or sp) of all elements except for hydrogen in the molecule to the right. (The first atom is done for you. ) H H H C N H H C O O C C C Cl H H C N sp3 9) When any two orbitals overlap and make a covalent bond, the orbitals either overlap as a sigma bond () or as a pi bond (). Describe which type of bond (, , or none) is being represented by the orbital overlaps in the following situations: a) b) c) d) px px sp3 sp2 s sp pz pz px pz e) 10) Label all of the orbitals (s, p, sp3, sp2, or sp) in these orbital diagrams and label all bonds as sigma () or pi (). a) Orbital representation of H H H H C C C H H b) Orbital representation of H C N c) Orbital representation of H Cl O C 11) Draw an orbital diagram for each of the molecules below. To do so, do the following steps: On the structure given, label the hybridization of each atom in structure (sp3, sp2, or sp) Draw the outline of the orbital diagram for the molecule. Make sure to draw the right number of hybrid orbitals around each atom and draw them oriented at correct angles. (For example, if an atom is tetrahedral, it is sp3 hybridized and so there are four sp3 orbitals oriented tetrahedrally.) Don’t forget the needed “p orbitals” for sp2 and sp hybridized atoms.) Label the diagram with the correct types of orbitals-- sp, sp2, sp3 or p. Label all sigma () and pi ( bonds. Put in all lone pairs. a) b) H N CH3 C N C Br O H