CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams
advertisement

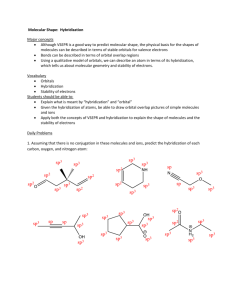
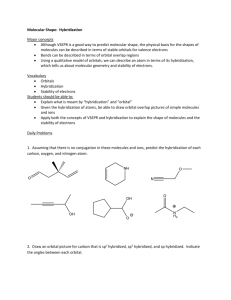
CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams - KEY 1. Draw 3-D orbital structures for each of the following. a. Water sp3 O H O H H H s H2O b. Ammonia H H N sp3 N H H H H NH3 c. Methyl cation + CH3 H H sp2 + C H H + C H H empty, unhybridized p orbital d. Methyl anion CH3 H lone pair in sp3 orbital 3 sp - C H H H H H e. Ethene C2H4 Page 1 of 5 CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams - KEY unhybridized p orbitals on Cs overlap above and below plane of molecule to form π bond H H H H H both Cs sp2 H H H σ + π = double bond f. Carbon dioxide CO2 lone pairs in sp2 orbitals sp O O O sp2 O 2 π bonds perpendicular to each other, due to overlap of unhybridized p orbitals between C and each O. g. Nitric acid HNO3 sp2 sp3 O O + O - + sp2 in both N H N - O O sp3 both resonance structures O sp3 in both sp2 1 H will draw all atoms sp2 to satisfy 2 O O O - N O + - N + H H O O 1 2 h. Formic acid Page 2 of 5 CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams - KEY HCOOH O O H H - H O C sp2 in both O sp3 + H sp2 1 2 O O H H H H O O 1 + 2 i. Hydrocyanic acid HCN H N H sp lone pair in sp orbital N σ + 2 π bonds = triple bond j. HCNO (noncyclic and connected as shown) Best resonance structures* + H N sp H O 3 sp sp N O + H - - sp H + C 2 N O sp sp2 N O + - lone pairs in sp2 orbitals and unhybridized p orbital on O Page 3 of 5 CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams - KEY *It would actually be best to show the O as being sp hybridized so that a pi bond could be formed in the y-plane as well as the z-plane in which case only one lone pair would be in an sp hybrid orbital and the other two would be in unhybridized p orbitals. k. Also try hydronium ion, CH3CH2NH- and CH2CN-, nitrite ion l. Tougher ones: ethyl radical, propene, acetylene Additional Information: 3-D orbital structures/diagrams are used to show the orbitals & e- occupancies for atoms in more detail than Lewis-Kekule structures. BE DETAILED! Steps for Drawing 3-D Orbital Structure: 1. Draw Lewis-Kekule structure. a. Include resonance structures where appropriate 2. Determine hybridization on EACH atom For atoms in the second period of the PT, we have the following orbitals s px to mix/hybridize Mix Hybridization s and px sp pz Hybrid orbital (# of them) (2) ║to paper py Bond angles Geometry 180o Linear 120o Trigonal Planar s and px and py sp2 (3) ┴ to paper s and px and pz (3) Page 4 of 5 CHEM 109A CLAS Drawing 3-D Orbital Structures/Diagrams - KEY s and px , py and pz sp3 109.5o * Tetrahedral (4) *Notable exceptions that will be assumed to be tetrahedral water w/ bond angle ~104o ammonia w/ bond angle ~107o a. hybridization on an atom must be the same in all resonance structures, so chose least hybridized. For example, if an O is sp2 hybridized in one resonance structure and sp3 hybridized in another, you should draw it as being sp2 hybridized in both. 3. Starting with the more central atom draw the 3-D orbital diagram a. Single lines represent sigma bonds and can be solid (in plane of paper), dashed (away from you, into paper) or wedged (towards you, out of paper). b. Include unhybridized orbitals (usu. p orbitals) on each atom since is it overlap of these orbitals that creates pi bonds. c. Place lone pairs as paired up and down arrows in orbitals (usu. hybridized, but sometimes unhybridized p orbitals). 4. Count up number of e-s – should equal the number of e-s in your Lewis-Kekule structure (#1). Page 5 of 5