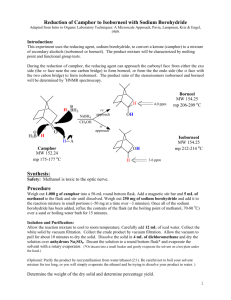
Borneol oxidation/sublimation
advertisement

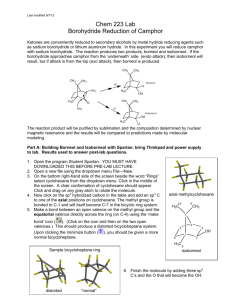
A ‘GREEN’ MICROWAVE OXIDATION1 Concepts of environmental stewardship pervade out culture and society but are often noticeable absent in chemical education. In recent years more chemical educators and those in the chemical industry are adopting practices that are ‘greener ‘or more environmentally friendly. Green chemistry is that which is ‘benign by design, can best be understood by considering the “Twelve Principles of Green Chemistry’ as outlined by Paul Anastas and John Warner in 1998. THE 12 PRINCIPLES OF GREEN CHEMISTRY 1. It is better to prevent waste than to treat or clean up waste after it is formed. 2. Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product. 3. Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment. 4. Chemical products should be designed to preserved efficacy of function while reducing toxicity. 5. The use of auxiliary substances (solvent etc) should be made unnecessary wherever possible and, innocuous when used. 6. Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperatures and pressure. 7. A raw material of feedstock should be renewable rather than depleting wherever technically and economically practicable. 8. Unnecessary derivatization (blocking groups, protection/deprotection) should be avoided whenever possible. Make the synthesis short and sweet. 9. Catalytic reagents are superior to stoichiometric reagents. 10. Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degraded products. 11. Analytical methodologies need to be further developed to allow for real-time, inprocess monitoring and control prior to the formation of hazardous substances. 12. Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions and fires. See the web site: www.chemistryorg/education/greenchem for more information about green chemistry. 1 This lab was written and developed by Craig Schwartz, March Sala and Rich Gurney of Northwestern University and Simmons College In our lab we are going to carry out an oxidation of borneol to camphor. This is a classic organic reaction and traditionally there are 2 methods that this oxidation can be carried out. On page 266 (exp 28) of PLKE text you have one such method of using sodium hypochlorite (bleach) in acetic acid solvent (see reaction #1 below). Another method is shown on page 850 of your Bruice organic text where alcohols are oxidized to ketones using chromic acid and sulfuric acid (#2 below). On the last page of this report is an experimental procedure for the chromic acid oxidation from an earlier addition of PLKE. In this lab we will carry out an oxidation using manganese dioxide/silica gel catalyst (#3 below) which is considered a ‘green’ reaction NaO Cl 1. CH3 CO 2 H HO O Borneol Camphor N aCr 2 O7 2. H 2 SO4 HO O Borneol Camphor MnO2 3. Silca gel HO Borneol O Camphor Pre-lab Before lab begins we want to assess the ‘greenness’ of the 3 reaction schemes shown above. The procedures for reactions #2 and #3 are on the following pages and for reaction #1 you will find the procedure on pg 266 of you PLKE text. To address the issue of greenness consider the following questions (#1-4 on the next page) of each reaction. For each question, consider which of the 12 green chemistry principle(s) it is addressing. Note: You don’t have to answer these questions to turn in, but we’ll talk about them in lecture next Tuesday. However, be sure to write in your lab notebook the purpose, the reaction equation and materials and methods for reaction #3 (above) and, in the spirit of Green chemistry, arrive to campus on lab night by foot, bus or bike. I’m kidding about that last bit; this campus is terrible to arrive by those methods after dark. 1. What is the toxicity of all of the chemicals used in the reaction? You can reference the Toxicity of a reagent by referring to the Material Safety and Data sheets (MSDS) for all the reagents. Use the web site http://hazard.com/msds/index.php to obtain an MSDS of a given chemical. You need only refer to the ‘highlights’ of the MSDS and the SAF-TDATA ratings if they are listed on the sheet (these are Health Flammability, Reactivity and Contact ratings that are given a 1-4 numerical value) 2. Are the catalytic reagents being used?(a catalyst is a reagent that increases the effective rate of the reaction without being itself consumed) In reaction (#3), manganese dioxide on silica gel is used as a catalyst. 3. What auxiliary substances, such as solvent, drying agent, etc. are needed? This can be a major issue as these auxiliary substances are often needed in great abundance creating high cost and waste issues. 4. Can we minimize the energy required for this reaction? A ‘hidden’ environmental and economical cost is often the energy used in a reaction. Heating and cooling requirements are a form of energy expenditure and should be reduced when possible. This is especially true for large scale industrial reactions. Procedure for the Green chemistry oxidation of borneol to camphor This procedure entails using manganese dioxide supported on silica gel as a catalyst and use of a microwave oven as the heating source. You will monitor the reaction using TLC. Upon completion, you will obtain the IR spectrum of your final product to determine if the alcohol was completely converted into the corresponding ketone. Procedure: 1. Add MnO2 (about 0.07 g), silica gel (about 1.2g) and borneol (about 0.3 g — record this weight exactly) to a pestle and grind with the mortar until a homogenous mixture is obtained (grind for about 5-7 minutes). When you have completed the lab, clean the mortar and pestle with 1 M HCl. 2. Transfer the sample into the bottom of a Petri dish. Weigh the Petri dish cover, then place it on top of the bottom of the dish. Put the covered dish in the center of a microwave oven. Microwave on high power for 5 intervals of 20 seconds each. Between intervals, turn the microwave off for 20 seconds. Once the five intervals are complete allow the Petri dish to cool. You will find that your product will have sublimed on to the Petri dish cover (make an observation of what the product looks like). Note: this time was optimized for the small microwave oven in the class; other microwave ovens will have different times, depending on the power of the oven — ask your instructor. 3. Collect a few crystals (I mean only a couple — you don’t want to artificially lower your yield calculation in step 5) from your Petri dish cover and dissolved in 10 drops of diethyl ether. Spot this solution on a TLC plate along with a 2% solutions of borneol and camphor (in ether) as a reference. Develop the plate in 90:10 hexane/ethyl acetate (which you will have to make up yourself — you’ll need about 20 mL total volume). 4. Visualize your TLC plate using an iodine chamber. Place your plate in the chamber and gently roll the chamber sideways until your plate is coated with the silica/iodine powder and your spots turn brown. If borneol is still present in your reaction, scrape the crystals from the cover into the mixture, regrind, and perform the heating process again. 5. Once the reaction is complete, weigh the Petri dish cover to acquire the weight of the product and collect the crystals in a sealed vial (to avoid sublimation). Calculate your percent yield. 6. Take an IR spectrum of your product. Since camphor is a solid you must first dissolve a few crystals of it in a few drops of methylene chloride and then pipet this solution onto one (cleaned) salt plate. Let the methylene chloride solvent evaporate and it will leave a think film of camphor that can then be analyzed. Do not worry about covering this with the second salt plate; simply slip the one plate in the holder.