CHEM-202L-Camphor
advertisement

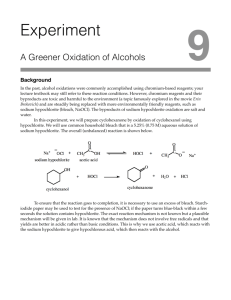
CHEM 202L Name: ________________________________ Oxidation of Borneol Ha OH NaOCl H CH3COOH Borneol O Cl H O H slow O Camphor Procedure: To a 50 ml round-bottom flask, add (0.360 g) of Borneol, (1.0 ml) acetone, and (0.30 ml) of glacial acetic acid. Attach a water condenser and place the round-bottom flask into a water bath at about 50C. (Use a 150 ml beaker for the water bath). It is important that the temperature of the water bath remain at 50C during the entire period. Addition of Sodium Hypochlorite: Measure out (6 ml) of 6% hypochlorite solution in a 10 ml graduated cylinder. Use a Pasteur pipette to drop wise add (0.5 ml) of the sodium hypochlorite solution every 4 minutes through the top of the water condenser. The addition will take 48 minutes to complete. Continue to stir and heat the mixture during the 48-minute period. After the addition, heat and stir the mixture for an additional 15 minutes. Extraction of Camphor: When the reaction time is complete, allow the mixture to cool to room temperature. Remove the water condenser, transfer the mixture to a test tube and add (3 ml) of dichloromethane to extract the camphor. Cap and shake well. Remove the organic layer and add another (3 ml) aliquot of dichloromethane to the aqueous layer to remove any residual product. Shake, separate, and remove the organic layer. Repeat one more time. Combine all three dichloromethane layers in a test tube and wash with (3ml) of aqueous saturated sodium bicarbonate solution. Stir till the bubbling ceases, cap and shake. Use a Pasteur pipette to remove the upper aqueous layer. Wash the organic phase with (3 ml) aqueous sodium bisulfate. Siphon off the top aqueous layer and perform a last wash with (3 ml) of water. Transfer the organic layer to a beaker, taking care to rinse the test tube with a small volume of dichloromethane. Dry the “goodies” with anhydrous sodium sulfate. Transfer the dichloromethane to a preweighed (50 ml) Erlenmeyer flask. Carefully, evaporate the solvent on a steam batch and recover the camphor. Calculate percent yield Analysis: Take a melting point (172-174C) Write your discussion on the back of this paper.