Lab #1: Borneol Oxidation
advertisement

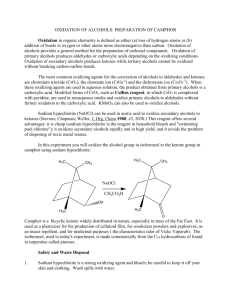
CHEMISTRY 344 - Organic Chemistry Laboratory II – Spring 2016 Lab #1: Oxidation of Alcohols to Ketones (Borneol Oxidation) Purpose. In this lab you will learn about oxidation reactions in organic chemistry, and apply this reaction to the oxidation of a secondary alcohol (borneol) to a ketone (camphor) using hypochlorous acid. You will purify this product by extraction and recrystallization, determine purity by mp, and measure reaction efficiency by calculating % yield. You must have lab goggles and your required Hayden-McNeil Student Lab Notebook. Each Team will work in pairs, students may split up the work into assigned tasks, BUT your entire Team should be comparing and discussing your approaches, results, and lab safety! Complete your lab notebook prelab work as outlined in the lab syllabus and below: (notebook items I – V). I. Your name and the name of your lab instructor. II. Title of the experiment and date. III. Background information and notes from the pre-lab assignment. Since this is the first lab of the spring semester, you will be given time to complete the pre-lab work during recitation. However, if you want to get out of lab earlier, you should try to get as much of the prelab prep work done before you come to lab. You are expected to read this lab handout before going to lab and to read about alcohol oxidation in a textbook or online. Then you should prepare as much of the following before lab as you can. You will learn more about the chemistry and experiment in the pre-lab recitation. There will be a group quiz for this lab based upon the handout. 1) Sketch the reaction from the handout in your notebook (a mechanism is not required) 2) In this lab you will review the lab techniques of (a) reflux using an air condenser, (b) extraction, (c) drying agents, and (d) melting point determination. Write a 1-2 sentence summary that describes the use of these techniques in the organic chemistry laboratory. 3) Write a short paragraph to detail the purpose and goals of this experiment (in your own words). 4) Research and write a few sentences about safety concerns associated with using bleach as a reagent in the organic laboratory. IV. Table of Reagents. Synthesis experiments require a table of all reagents, including the chemical name, formula, molecular weight, molecular structure, and physical properties (melting point, boiling point, density). You should record information for borneol, camphor, acetone, and acetic acid. Also include information for bleach (dilute sodium hypochlorite / hypochlorous acid) if possible. Use the pre-lab recitation period to fill in any information that you are unable to locate on your own. CHEMISTRY 344 - Organic Chemistry Laboratory II – Spring 2016 Lab #1: Oxidation of Alcohols to Ketones (Borneol Oxidation) Background. A simple oxidizing reagent for the preparation of ketones from secondary alcohols is sodium hypochlorite (NaOCl) in acetic acid. Sodium hypochlorite is the active agent in commercial bleach, and it oxidizes secondary alcohols rapidly and in high yield. The actual oxidizing agent, hypochlorous acid (HOCl), is made by reacting sodium hypochlorite with acetic acid. A general scheme for the reaction is shown below: Experimental Procedure. Step 1: Setting up the Reaction Mixture. To a 10 ml round bottom flask, add 0.360 g racemic borneol, 1.0 ml acetone and 0.30 ml glacial acetic acid. Add a magnetic stir bar to the flask, attach an air condenser, and place it in a water bath at around 50oC. Use a 150 ml beaker for the water bath that you heat directly on a hotplate. Note that poor yields will result if your water bath temperature is too low. If the borneol does not dissolve, add an additional 0.50 ml of acetone. Step 2: Addition of NaOCl (Be sure to read Step 3 before you start this step). Measure out 7 ml of 6.5% NaOCl solution in a 10 ml graduated cylinder. With a Pasteur pipet, add dropwise 0.5 ml NaOCl every 4 minutes through the top of the air condenser as you continue stirring and heating the reaction mixture. After the last addition, heat and stir the mixture for an additional 15 minutes. Step 3: Extraction of Camphor. When the reaction period has completed, let the reaction mixture cool to room temperature. Remove the air condenser, transfer the reaction mixture to a screw cap centrifuge tube and add 2.0 ml of methylene chloride (CH2Cl2). Cap the centrifuge tube and mix, venting from time to time. If the layers do not separate, add an additional 2 ml of CH2Cl2 and mix again. Using a Pasteur pipette, transfer the CH2Cl2 layer to another centrifuge tube. Extract the reaction mixture with a second, 2 ml portion of CH2Cl2, and combine with the first CH2Cl2 solution. Wash the combined CH2Cl2 solution with 2.0 ml saturated aqueous sodium bicarbonate (NaHCO3) solution. Stir the liquid until the bubbling produced by the formation of CO2 ends. Cap the tube, shake, and vent to release any pressure. Transfer the lower CH2Cl2 layer to a new tube and wash with 2.0 ml of 5% aqueous sodium bisulfite (NaHSO3 ). Sodium bisulfite decomposes the excess oxidizing agent. Transfer the CH2Cl2 layer to another centrifuge tube and wash with 2.0 ml water. Remove the CH2Cl2 layer and transfer to a dry test tube. Step 4: Isolation of Camphor. Dry the CH2Cl2 layer over granular anhydrous sodium sulfate (Na2SO4). Transfer the dried CH2Cl2 layer to a pre-weighed 10 ml Erlenmeyer flask. Evaporate the solvent in the heated sand bath in the hood. After the solvent has evaporated a solid should appear. Take your Erlenmeyer flask and dry your isolated product in an oven at 110oC for 10 minutes. Record the appearance of your product, and determine its mass and mp. When determining the mp remember to heat the sample slowly. You can quickly bring the Mel-Temp up to within 300C of camphor’s expected mp, but the final 20-300 should be done at about 10C every 10 sec. The melting point of pure racemic camphor is 174oC and for borneol is 206-209oC. CHEMISTRY 344 - Organic Chemistry Laboratory II – Spring 2016 Lab #1: Oxidation of Alcohols to Ketones (Borneol Oxidation) V. Observations, Results and Data. You are not required to re-write the experimental procedure! All work in this section will be based on the actual experiment, and must be written in Table format. The required information for this section will include: all reagent mass and volume measurements, observations, crude and pure product mass or volume, and product analysis by melting point. Immediately following this Table you will also have: I. All calculations that are relevant to information in the Table. II. A sketch or diagram of any new or unusual experimental apparatus. VI. Discussion. Write a paragraph to addresses the techniques you used and observations that you recorded in this experiment. Address whether the experiment went according to the planned procedure or any deviations occurred. VII. Conclusions. A short paragraph is expected in which you directly address your data. Was your experiment a success considering the purpose, goals, and results of the experiment? Directly address the % yield and your melting point data. What can you state about the efficiency of this reaction and your experimental technique? If your Team Discussion is not finish by the end of the lab period, you should all go to a lounge area and complete your work. You can slide your stapled notebook pages under the door of the Chemistry Department Office, room CON 401. The chemistry administrative assistant will place your lab reports into your lab instructor’s mail box.