Reduction of Camphor to Borneol
advertisement

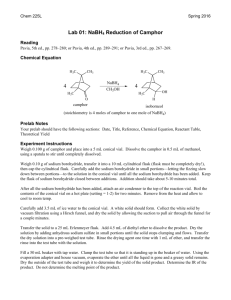
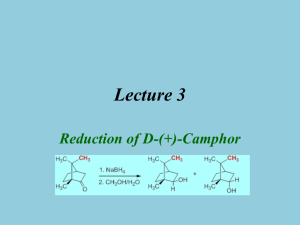
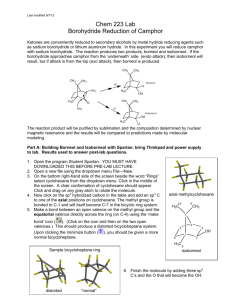
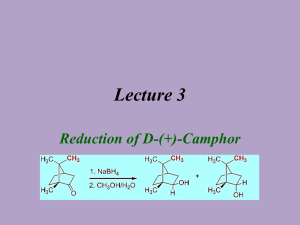
Reduction of Camphor to Isoborneol with Sodium Borohydride Adapted from Intro to Organic Laboratory Techniques: A Microscale Approach, Pavia, Lampman, Kriz & Engel, 1989. Introduction: This experiment uses the reducing agent, sodium borohydride, to convert a ketone (camphor) to a mixture of secondary alcohols (isoborneol or borneol). The product mixture will be characterized by melting point and functional group tests. During the reduction of camphor, the reducing agent can approach the carbonyl face from either the exo side (the re face near the one carbon bridge) to form borneol, or from the the endo side (the si face with the two cabon bridge) to form isoborneol. The product ratio of the stereoisomers isoborneol and borneol will be determined by 1HNMR spectroscopy. H H BH3 re si H3B H NaBH4 re approach 4.0 ppm Borneol MW 154.25 mp 206-209 oC OH CH3OH si approach O Isoborneol MW 154.25 mp 212-214 oC H A Camphor MW 152.24 mp 175-177 oC OH H 3.6 ppm Synthesis: Safety: Methanol is toxic to the optic nerve. Procedure Weigh out 1.000 g of camphor into a 50-mL round bottom flask. Add a magnetic stir bar and 5 mL of methanol to the flask and stir until dissolved. Weigh out 250 mg of sodium borohydride and add it to the reaction mixture in small portions (~50 mg at a time over ~3 minutes). Once all of the sodium borohydride has been added, reflux the contents of the flask (at the boiling point of methanol, 70-80 0C) over a sand or boiling water bath for 15 minutes. Isolation and Purification: Allow the reaction mixture to cool to room temperature. Carefully add 12 mL of iced water. Collect the white solid by vacuum filtration. Collect the crude product by vacuum filtration. Allow the vacuum to pull for about 10 minutes to dry the solid. Dissolve the solid in 4 mL of dichloromethane and dry the solution over anhydrous Na2SO4. Decant the solution to a round bottom flask* and evaporate the solvent with a rotary evaporator. (*Or decant into a small beaker and gently evaporate the solvent on a hot plate under the hood.) (Optional: Purify the product by recrystallization from water/ethanol (2:1). Be careful not to boil your solvent mixture for too long, or you will simply evaporate the ethanol and be trying to dissolve your product in water. ) Determine the weight of the dry solid and determine percentage yield. 1 Characterize your product and compare to camphor starting material by A. “wet chemistry” methods: melting point, (pure racemic isoborneol melts at 212oC) Functional Group tests for alcohols and ketones (see below) B. Spectroscopic methods: IR 1 H-NMR Functional Group Tests Two classical tests will be performed in order to determine whether or not the product is an alcohol and whether or not it is contaminated by unreacted camphor. For procedures a and b below, record your observations and results in tabular form and give general equations for positive tests. a. Chromic Acid Test for Alcohols: In the chromic acid test, 1° and 2° alcohols are oxidized by solutions of chromic acid (H2CrO4) to form carboxylic acids and ketones, respectively; tertiary alcohols do not react with H2CrO4. A positive test is indicated by the formation of a green-blue precipitate. Add a match head size of solid or 1 drop of liquid to 1 mL acetone in a test tube. Then add 1 drop of chromic acid reagent (a orange color) and shake the mixture. The appearance of an opaque blue-green color within 2 seconds is a positive test. Perform the test on 1-butanol, 2-butanol, 2methyl-2-propanol (t-BuOH), camphor, and your product. b. 2,4-Dinitrophenylhydrazine (2,4-DNP) Test for Ketones: In the 2,4-DNP test, ketones (and aldehydes) react rapidly with 2,4-dinitrophenylhydrazine to give brightly colored precipitates. Alcohols do not react with this reagent. Different concentrations of reactants will be used to increase the sensitivity of the test, so that small amounts of unreacted starting material (camphor) can be detected in the product from your reduction reaction. Knowns Perform this test on the following substances: isoborneol, camphor, methanol, and acetone. For each test, put 1 mL of 95% ethanol in a small test tube. Add (and mix) a spatula-tip full or 5 drops of the test compound, followed by 1 mL of 2,4-DNP reagent. Mix well. If a precipitate does not form, boil the solution in the test tube in a steam bath for 1 minute. Cool it in ice. A colored precipitate is a positive test for a ketone. Reaction product Repeat the 2,4-DNP test as above, but use about 0.1 g of your product. If a positive test is observed, explain why this occurred. Write-Up/ Formal Report: Besides the standard information in a formal report be sure to include: Information and background about camphor Wet chemistry proof of reaction from analysis of starting material and products. Interpretation of the IR spectrum of your product. Describe the presence and absence of any significant IR absorptions for the starting material and products that indicate that a reaction has occurred? Interpretation of the 1H-NMR spectrum of your product. Determine the ratio of isoborneol to borneol from your 1H-NMR spectrum (the downfield resonance for the CH-OH of borneol occurs at 4.0 ppm and for isoborneol at 3.6 ppm). Include copies of any spectral data with structures and interpretation. Note the presence of any unreduced starting material in the product and tell how you determined this. Discuss why the major product of this reduction is isoborneol and not borneol. 2 Questions: Prelaboratory Questions: 1. Draw out the step by step mechanism of the reduction of camphor with sodium borohydride showing the expected stereochemistry of the products. Label the products as having been formed from exo approach or endo approach. 2. How might the geometry of the product change (OH in an endo or exo position?) if all the methyl groups of camphor were replaced with H? 3. The reduction mechanism is often shown with a hydride (H-) ion attacking the carbonyl carbon. Why might one assign a partial negative charge to the hydrogens in sodium borohydride? 4. Explain the comparative reactivity and hazards of NaBH4 as compared to LiAlH4. Also, compare the different results when they are added to A) water; B) ethyl acetate; C) methanol. 5. A mixture of borneol and isoborneol gave the following 1HNMR signals with the given integrations (2.314 and 9.497). Determine the product ratio of isoborneol to borneol in this particular mixture. 6. Predict the product of the following reduction reactions: a. O O LiAlH4 O NaBH4 CH3CH2OH CH3CH2OH b. O O NaBH4 H O CH3OH c. O H CH3 NaBH4 CH3OH H3C OCH3 Post lab Extra Credit: The reduction in this experiment gives largely isoborneol to the exclusion of borneol. Starting from camphor, provide a multi-step synthesis that would favor the formation of borneol over isoborneol. 3 Infrared Spectra of: Camphor, Borneol, and Isoborneol 4