Chem 30BL-Lecture 3
advertisement
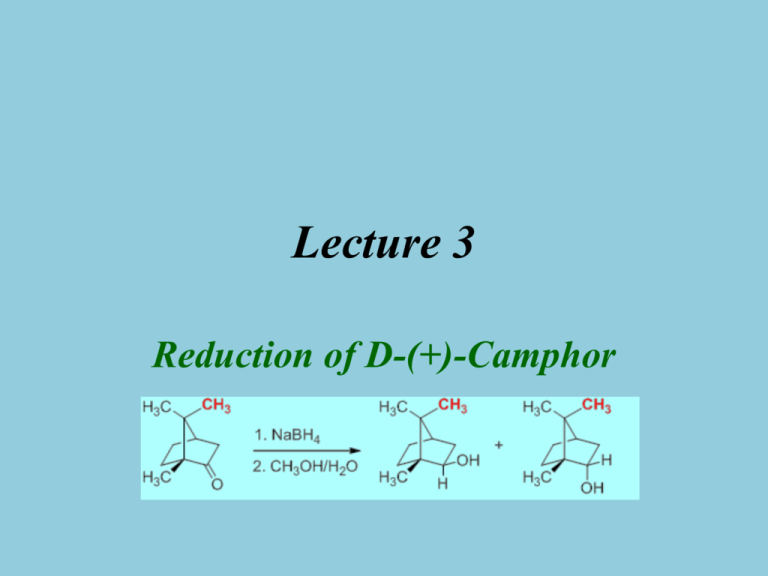
Lecture 3 Reduction of D-(+)-Camphor Introduction • Reduction of Ketones and Aldehydes Reactant Ketone Product Alkane Ketone Ketone Ketone Aldehyde Ketone Alcohol Alcohol Alcohol Alcohol + Acid Diol Reagent Name Zn/HCl N2H4/KOH H2/Ni Al(OCH(CH3)2)3 NaBH4, LiAlH4 KOH Mg-metal hn/(CH3)2CHOH Clemmensen Wolff-Kishner Raney Nickel Meerwein-Ponndorf Cannizzaro Pinacol Pinacol for aromatic ketones Mechanism I • In Chem 30BL, sodium borohydride (NaBH4) will be used as the reducing agent • Driving force for reaction is the formation of a very strong B-O bond vs. the p-bond of the carbonyl group and the B-H bond • The light elements of group 3 often form compounds that possess a partial double bond character in the E-X bond, if X has one or more lone pairs i.e., N, O, F, etc. Mechanism II – H BH3 R2C O H BH3 R2C O O 3R C R (occurs 3 mo re times) Keton e o r Aldeh yde – R2 CHO B(OCHR2 )3 OH 4 R C R H H O CH3 + (CH3O)4B –Na+ (large ex cess) (deco mp oses at R H C R H O R – R C O B O C R R O H H C R R "tetraalkyl b orate" elevated temp eratures) • Ultimately, two hydrogen atoms are added to the ketone: one originates from the hydride (:H-), which forms the C-H function, and the other one from the protic solvent (H+) that leads to the formation of the hydroxyl function Mechanism (Stereochemistry I) • The reduction of 2-pentanone affords a racemic mixture of 2-pentanol because the activation energies (G‡) for the two alternate pathways are identical • The reduction of D-(+)-camphor affords a mixture of two diastereomeric alcohols. The exo product (=(-)-isoborneol) is formed in larger quantity compared to the endo product (=(+)-borneol) because the activation energy for the formation of the exo product is lower Mechanism (Stereochemistry II) • The stereochemistry of the reaction can be explained using HOMO-LUMO concept – The hydride is the nucleophile in the reaction which provides the electrons for the newly formed C-H bond – The carbonyl group is the electrophile in the reaction and therefore has to provide an empty orbital for the reaction (p*(C=O), LUMO) p p-bond p-bond exo approach C 100-120o C O C O O p endo-approach HOMO of a C=O bond (side view) LUMO of a C=O group (side view) The p-orbital is closer to the oxygen atom, hence the oxygen contributes more to the orbital (bigger lobes). In the p*-orbital the situation reverses. Mechanism (Stereochemistry III) • Exo approach camphor • Endo approach camphor Exo approach 2-norbornanone Bottom line: – Camphor: exo approach is sterically more hindered resulting in a higher activation energy for this pathway and a lower quantity of the endo product (=borneol) – 2-norbornanone: the exo approach is less hindered resulting in the endo product as major product Mechanism (Stereochemistry IV) • The stereoselectivity for the reaction would be higher – if the R-group on the side of the carbonyl function was increased in size – if the size of the nucleophile was increased Reducing agent NaBH4 LiAlH4 LiAlH(OMe)3 LiBH(n-Bu)3 LiBH(sec-Bu)3 LiBH(iso-amyl)3 2-Norbornanone (endo product) 86 % 89 % 98 % 98 % 99.6 % >99.5 % Camphor (exo product) 86 % 92 % 99 % 98 % 99 % 99.3 % – if the reaction temperature was lowered Experimental Design • Choice of Reducing Agent – LiAlH4 : causes a high stereoselectivity, more reactive (can even be pyrophoric); requires very dry diethyl ether or very dry tetrahydrofuran as a solvent – NaBH4 : much safer but strong enough of a reducing reagent to reduce the ketone • Choice of Solvent – NaBH4: moderately soluble in water, insoluble in diethyl ether – Camphor: very poorly soluble in water (0.1 g/100 mL), well soluble in diethyl ether – The solvent choice is a compromise in terms of polarity: methanol dissolves both compounds reasonably well (NaBH4: 13 g/100 mL, camphor: 63.1 g/100 mL) – Problem: Sodium borohydride reacts with protic solvents – Solution • A large excess of the reducing agent is used to ensure the complete reduction of the camphor • Camphor is dissolved in a small amount of methanol before the NaBH 4 is added, which takes advantage of the fact the reduction of the camphor is faster than hydrolysis of NaBH4 Experiment I • Dissolve the camphor in a small amount of methanol in a 25 mL Erlenmeyer flask • Add the sodium borohydride in three portions Watch glass with ice Boiling stick of appropriate length • Bring the suspension to a gentle boil • After the reaction is completed, place the solution in a cold water bath • What is the setup here? • Why? • Add ice-cold water to the reaction mixture • Isolate the solid using vacuum filtration • Why is water added? • Suck air through the solid for at least 10 minutes • Why is air sucked through the solid? To have better control over the reaction during the addition of the water To complete the hydrolysis and to precipitate the organic compounds (1.2 mg/mL borneol in water at 25 oC) To remove the bulk of the water from the solid Experiment II • Dissolve the solid in a small amount of diethyl ether • Add a small amount of drying agent (MgSO4) • Why is the solid dissolved again? • Remove the drying agent • Extract the drying agent with a small amount of diethyl ether • How is accomplished? • Why is this step necessary? In order to dry it • What is the student looking for here? 1. Some free floating drying agent 2. A transparent solution To recover some of the adsorbed product • Why is the drying agent removed? 1. The drying process is reversible 2. The product and the drying agents are both white solids which makes it impossible to separate them later • Remove the solvent using the rotary evaporator • Why is the rotary evaporator used? • How this piece of equipment work? See video for details Characterization I • Melting point (~ 1 mm in melting point capillary) – Too much sample will result in a broader melting point range • Infrared spectrum • Isoborneol (KBr): – n(OH)=3398 cm-1 (broad peak) – n(C-OH)=1069 cm-1 (strong) – n(C=O)=1744 cm-1 is absent! n(C=O) n(OH) Isoborneol n(C-OH) Borneol n(C-OH) • Borneol (KBr): – n(OH)=3352 cm-1 (broad peak) – n(C-OH)=1055 cm-1 (strong) n(OH) Characterization II • Gas chromatography – Prepare a solution of the final product in diethyl ether (conc: ~1 mg/mL) – Fill the GC vial to the 1.5 mL mark – Close the vial with a cap and submit into tray – The sample cannot contain any undissolved solids or water because they will cause significant problems during the data acquisition – Sign the sample in on the sign-in sheet: student name and code on the vial (make sure not to remove it). Do not forget to record the code in your notebook as well. – Samples that are not signed in will not be run! – Pick up the printout in YH 3077E during the afternoon of the next day Polarimetry I • Optical activity was discovered by E.L. Malus (1808) • Chiral molecules rotate the plane of polarization of polarized light Polarizer Analyte Analyzer • How does it work? – Monochromatic light is polarized by a Nicol prism (polarizer) – The plane-polarized light passes through a polarimetry cell in which the plane of the light will be rotated if the cells contains a chiral compound (or a mixture of chiral compounds) – The analyzer rotates the plane of the light back to its original orientation Polarimetry II • The value of the optical rotation (a) of a sample depends on the wavelength (the subscript “D” refers to l=589.3 nm), the path length (l), the concentration (c) and the specific optical rotation for the specific enantiomer and to a lesser degree on the temperature (X) []X D *c*l • The sign of the optical rotation is independent from the absolute configuration! • The sign and absolute value can depend on the solvent because the observer might look at different compounds i.e., cation, anion or neutral specie for amino acids. Polarimetry III • Polarimeter (located in YH 1096 for Chem 30BL) • Concentration: ~1 % in 95 % ethanol (the exact concentration in g/mL has to be known) • It is important that there are no air bubbles in the path of the light because they will cause problems in the measurement (i.e., dark sample error) • The ratio of (-)-isoborneol and (+)-borneol can be calculated by • =x(-34.6o)+(1-x)(+37.7o) =specific optical rotation of the sample after concentration correction x =the mole fraction of isoborneol in the sample []D= +37.7o for (+)-borneol and []D= -34.6o for (-)-isoborneol Polarimetry IV • What influences the result in the polarimetry measurement? – The concentration of the sample – A wet sample will yield a less negative value because the concentration is less than assumed, which results in a lower reading for the sample – The presence of unreacted camphor ([]D= +44.26o ) – The ratio of the (-)-isoborneol and (+)-borneol i.e., a 80:20 mixture should result in a value of []= ~ -20o after the concentration correction