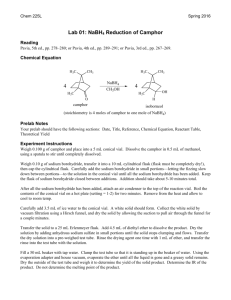
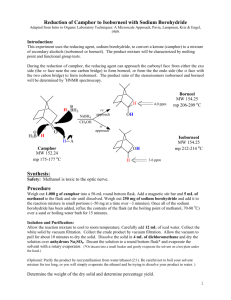
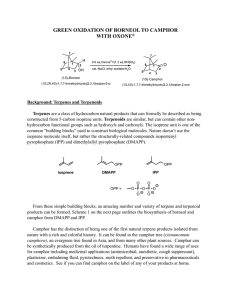
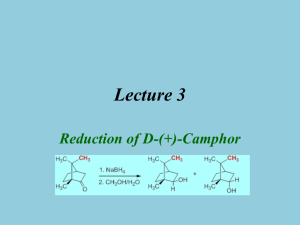
Reduction-camphor
advertisement

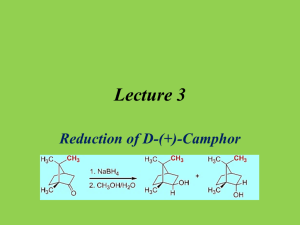
Name ____________________ Experiment #4 Section___________________ Date______________________ Reduction of Camphor Equation – Draw the overall equation for the reduction of camphor by sodium borohydride. Mechanism – In the space provided below show a detailed arrow pushing reaction for the reduction of camphor. DataWeight of camphor ___________________ Weight of sodium borohydride __________________ Weight of Product Crude _________________ M.P. Of Product ____________________ JVM v1.0 3/4/12 Observations- IR Attach a copy of your IR to this sheet. Fill in information for the relavant IR peaks in the chart below. Position (cm-1) Peak characteristics Bond type Functional group Based on your IR spectra comment on how well your reaction worked. H1-NMR Using the included NMR of the product from one of you classmates draw the structure of the product and asign the protons in this structure to peaks seen in the spectra. Calculations 1) Calculate the percent Yield for the formation of alcohol product in your experiment. Be sure to clearly label the calculations. JVM v1.0 3/4/12 2) Using the given H1-nmr calculate the ratio of the two diasteriomeric products formed in this reaction. Be sure to clearly label the calculations. Questions (1) Which diasteriomer is the major product? Why should this be the major product? (2) When the reaction is complete and acid is added the reaction fizzes. What gas is being given off? (3) What are commercial uses for the compounds encountered in this experiment (camphor, isoborneol and borneol)? JVM v1.0 3/4/12