GREEN OXIDATION OF BORNEOL TO CAMPHOR WITH OXONE
advertisement
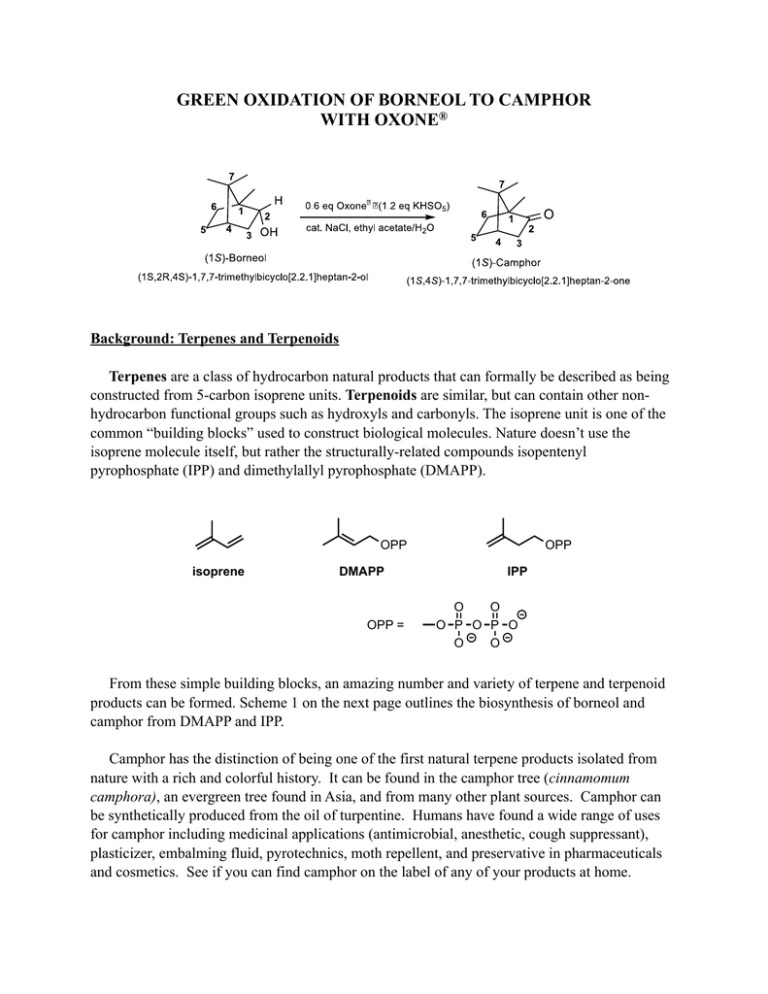
GREEN OXIDATION OF BORNEOL TO CAMPHOR WITH OXONE® Background: Terpenes and Terpenoids Terpenes are a class of hydrocarbon natural products that can formally be described as being constructed from 5-carbon isoprene units. Terpenoids are similar, but can contain other nonhydrocarbon functional groups such as hydroxyls and carbonyls. The isoprene unit is one of the common “building blocks” used to construct biological molecules. Nature doesn’t use the isoprene molecule itself, but rather the structurally-related compounds isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). OPP isoprene DMAPP OPP = OPP IPP O O O P O P O O O From these simple building blocks, an amazing number and variety of terpene and terpenoid products can be formed. Scheme 1 on the next page outlines the biosynthesis of borneol and camphor from DMAPP and IPP. Camphor has the distinction of being one of the first natural terpene products isolated from nature with a rich and colorful history. It can be found in the camphor tree (cinnamomum camphora), an evergreen tree found in Asia, and from many other plant sources. Camphor can be synthetically produced from the oil of turpentine. Humans have found a wide range of uses for camphor including medicinal applications (antimicrobial, anesthetic, cough suppressant), plasticizer, embalming fluid, pyrotechnics, moth repellent, and preservative in pharmaceuticals and cosmetics. See if you can find camphor on the label of any of your products at home. -H+ -PPO OPP DMAPP OPP OPP H IPP OPP OPP linalool synthase linalool pyrophosphate GPP H 2O -H+ [O] OH borneol O camphor Scheme 1: Biosynthesis of Borneol and Camphor The objective of this experiment is to oxidize (1S)-borneol to (1S)-camphor. This reaction has practical utility, because the (1S)-enantiomer of borneol is much cheaper than the (1R)- ($0.97/g vs. $95/g), but the (1S)-enantiomer of camphor is much more expensive than the (1R)- ($17/g vs. $0.36/g). Therefore, if the oxidation can be done cheaply and in high yield, it can provide a cheaper source of (1S)-camphor. The Reaction: Sodium hypochlorite (NaClO; household bleach) is a relatively cheap and environmentallyfriendly oxidizing agent capable of converting alcohols to ketones. In the presence of acid, hypochlorous acid is presumably formed, which could oxidize the alcohol via the following mechanism: However, household bleach can vary in concentration, diminishing over time. Also, its noxious vapors are an inhalation hazard. In this experiment, hypochlorite will be generated in situ by oxidizing chloride (from NaCl) with Oxone®. This reagent, a product of DuPont, is a very cheap, easy to handle, solid oxidant. Oxone® is a “triple salt” (2KHSO5.KHSO4.K2SO4) containing potassium peroxymonosulfate (KHSO5) as the active oxidizing agent. In this experiment, it us used to oxidize chloride to hypochlorite: KHSO5 + Cl- + H+ à KHSO4 + HOCl Because chloride is then regenerated when the alcohol reacts with hypochlorous acid, only a catalytic amount of chloride is needed for the reaction. Procedure3 Oxone® is a strong oxidant; do not inhale the dust. The aqueous components of an organic Oxone® reaction are oxidizing and acidic and should be quenched with sodium bisulfite and then neutralized with sodium bicarbonate before disposal. NOTE: carefully measure the amounts of solvent using a pipette. The rate-determining step of the reaction is second order, so the rate dramatically decreases with an increase in the solvent volumes. Your goal by the end of Day 1 is to have your crude product drying in a dessicator. On Day 2 you will purify your product by sublimation. Clamp a 50-mL round bottom flask to a ring stand and position a magnetic stirrer beneath it. Place a medium stir bar in the flask and add 1.0 g (6.5 mmol) of (1S)-borneol. Add 4 mL ethyl acetate and begin stirring to dissolve the borneol. Add 2.4 g (3.9 mmol) of Oxone® (which corresponds to 7.8 mmol of KHSO5) to the flask with continued stirring. Then add 0.08 g (1.4 mmol) NaCl, followed by 1.5 mL of deionized water. Allow the reaction to stir at room temperature for 50 minutes. Then add an additional 0.03 g (0.5 mmol) NaCl. Continue to stir for 10 more minutes. Note any changes in color or temperature during the entire procedure. By the end of this time the oxidation should be complete and excess oxidant can be destroyed. Add 15 mL of deionized water to the reaction and continue stirring to dissolve most of the salts. Slowly add a spatula tip of solid sodium bisulfite to reduce the oxidants that remain. Test the aqueous layer (not organic) by dipping a glass rod into it and then touching a piece of starchiodide paper. A blue-black color (positive test) indicates the presence of excess oxidant; add small amounts of sodium bisulfite if the aqueous layer tests positive, until a negative test is achieved (no color change). Workup Carefully transfer contents to a separatory funnel. Add 1 to 2 mL of ethyl acetate to the reaction flask, swirl, and add this wash to the separatory funnel as well. Shake and invert the separatory funnel and separate the layers by draining the aqueous layer from the bottom. Pour the organic layer out of the top into a clean 50-mL Erlenmeyer flask. Extract the aqueous layer twice more, with 5 mL of ethyl acetate each time. Return the combined organic phases to the separatory funnel and wash three times with 5-mL portions of saturated aqueous sodium chloride solution (brine). Pour the organic phase into a clean Erlenmeyer flask and dry over anhydrous sodium sulfate. Filter the solution into a tared Erlenmeyer flask. In the hood, heat gently on a hot plate and flow a light stream of air over the solution to remove the ethyl acetate solvent. Excessive heating or air flow can lead to sublimation and product loss, so it is important to monitor the concentration step carefully, especially near the end when the solid product begins to form. Record a crude mass before drying. Desiccation First, break up any large chunks of your product and/or material adhered to the walls of the flask to aid in desiccation. Label your flask with your name and “Camphor”. Cover the mouth of the flask with a Kimwipe using a rubber band, and place it in a CaCl2-containing dessicator. Rest the Erlenmeyer flask inside a small beaker to keep it upright. Next laboratory period, record the mass again to determine if excess water and/or solvent was removed. Set aside a small amount of crude product for IR and potentially NMR analysis. Sublimation Your TA will demonstrate how to set up a small-scale sublimation apparatus. Purify your crude camphor by sublimation. Collect the sublimed material onto a tared watch glass and obtain the mass. Save the material for your TA’s inspection. IR Interpretation Obtain IR spectra for your crude and final products. Qualitatively assess the extent of conversion in your crude product. Is your IR spectrum of your sublimed product consistent with what you would expect? 1H NMR Interpretation You will be provided with 1H NMR spectra for borneol, camphor, one student’s crude camphor product, and one student’s sublimed camphor product. Determine the percent conversion of borneol to camphor in the crude sample, and assess the quality of the crude and final product. It is not necessary to identify the chemical shift, splitting patterns, or coupling constants for every single proton of these compounds since the spectra are quite complex. However, by identifying signals that are diagnostic for each compound, and by integrating these signals, you can arrive at a ratio of product to starting material. Also look for any evidence of impurities such as ethyl acetate.