ELECTRON CONFIGURATION (SHORT FORM) n # of electrons in
advertisement
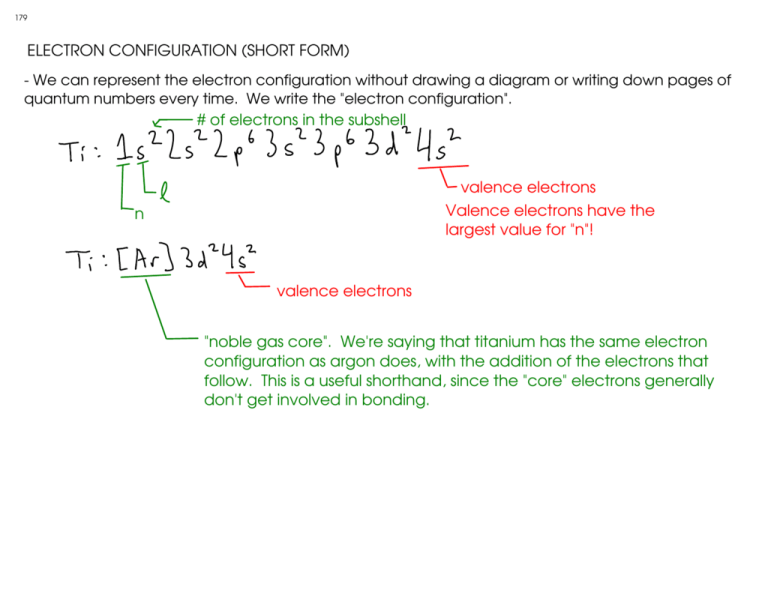
179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration". # of electrons in the subshell valence electrons Valence electrons have the largest value for "n"! n valence electrons "noble gas core". We're saying that titanium has the same electron configuration as argon does, with the addition of the electrons that follow. This is a useful shorthand, since the "core" electrons generally don't get involved in bonding. 180 ELECTRON CONFIGURATION AND THE PERIODIC TABLE IA H IIA Li Be IIIA IVA VA VIA VIIA He Na Mg IIIB IVB VB VIB VIIB K Ca Sc Ti Rb Sr Y Zr V Ra Ac Rf IB IIB VIIIB Cr Mn Fe Co Ni B C N O F Al Si P S Cl Ar Cu Zn Ga Ge As Se Br Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta W Fr VIIIA Re Os Ir Db Sg Bh Hs Mt Pt Au Hg Tl Sn Sb Te Pb Bi I "p" block: last electron in these atoms is in a "p" orbital! "d" block: last electron in these atoms is in a "d" orbital Kr Xe Po At Rn "inner" transition metals go here "s" block: last electron in these atoms is in an "s" orbital! Ne 181 - To write an electron configuration using the periodic table, start at hydrogen, and count up the electrons until you reach your element! IA 1 VIIIA H IIA 2 Li Be 3 Na Mg IIIB IVB VB VIB VIIB K IIIA IVA VA VIA VIIA He "d" block: The d block is shifted DOWN.! Ca Sc Ti Rb Sr Y Zr V Cr Mn Fe Co Ni Ra Ac Rf C N O F Al Si P S Cl Ar Cu Zn Ga Ge As Se Br Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta W Fr IB IIB VIIIB B Re Os Ir Db Sg Bh Hs Mt Example: Phosphorus (P): Noble gas core notation for P: Pt Au Hg Tl Sn Sb Te Pb Bi I Ne Kr Xe Po At Rn "inner" transition metals go here 182 EXAMPLES: F S Cl Remember - valence electrons are ALL of the electrons in the outermost SHELL (n)! More that one subshell (l) may be included in the valence electrons TITANIUM is a transition metal that commonly forms either +2 or +4 cations. The 4s electrons are lost when the +2 ion forms, while the 4s AND 3d electrons are lost to form the +4! You can order the subshells in numeric order OR in filling order Ti Se Noble gas core notation. Use the previous noble gas on the table, then add the electrons that it doesn't have to the end. Kr Sample f-block element PERIODIC TRENDS 183 - Some properties of elements can be related to their positions on the periodic table. ATOMIC RADIUS - The distance between the nucleus of the atoms and the outermost shell of the electron cloud. - Relates to the size of the atom. - As you go DOWN A GROUP ( ), the atomic radius INCREASES. - Why? As you go down a period, you are ADDING SHELLS! - As you go ACROSS A PERIOD ( ), the atomic radius DECREASES Why? Let's look at some sample atoms. lithium Outer electrons see an effective +7 charge (shielded from +9 nucleus by 2 electrons) Outer electron sees an effective +1 charge (shielded from +3 nucleus by 2 electrons) fluorine ... so fluorine's outer shell is pulled closer to the nucleus than lithium's! 184 (FIRST) IONIZATION ENERGY - The amount of energy required to remove a single electron from the outer shell of an atom. - Relates to reactivity for metals. The easier it is to remove an electron, the more reactive the metal. - As you go DOWN A GROUP ( ), the ionization energy DECREASES. - Why? As you go down a period, you are ADDING SHELLS. Since the outer electrons are farther from the nucleus and charge attraction lessens with distance, this makes electrons easier to remove as the atoms get bigger! - As you go ACROSS A PERIOD ( ), the ionization energy INCREASES. - Why? Let's look at some sample atoms. lithium Outer electrons see an effective +7 charge (shielded from +9 nucleus by 2 electrons) Outer electron sees an effective +1 charge (shielded from +3 nucleus by 2 electrons) fluorine ... since fluorine's outer electrons are held on by a larger effective charge, they are more difficult to remove than lithium's. LARGER IONIZATION ENERGY 185 THE FIRST TWO PERIODIC TRENDS IN A NUTSHELL IA VIIIA H IIA Li Be IIIA IVA VA VIA VIIA He Na Mg IIIB IVB VB VIB VIIB K Ca Sc Ti Rb Sr Y Zr V Ra Ac Rf LARGER SMALLER RADIUS IONIZATION ENERGY IB IIB VIIIB Cr Mn Fe Co Ni B C N O F Al Si P S Cl Ar Cu Zn Ga Ge As Se Br Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta W Fr SMALLER RADIUS Re Os Ir Db Sg Bh Hs Mt Pt Au Hg Tl Sn Sb Te Pb Bi I Ne Kr Xe Po At Rn "inner" transition metals go here 186 ELECTRON AFFINITY - the electron affinity is the ENERGY CHANGE on adding a single electron to an atom. - Atoms with a positive electron affinity cannot form anions. - The more negative the electron affinity, the more stable the anion formed! - General trend: As you move to the right on the periodic table, the electron affinity becomes more negative. EXCEPTIONS - Group IIA does not form anions (positive electron affinity)! valence electrons for Group IIA! period number - To add an electron, the atom must put it into a higher-energy (p) subshell. - Group VA: can form anions, but has a more POSITIVE electron affinity than IVA valence electrons for Group VA! Half-full "p" subshell! To add an electron, must start pairing! - Group VIIIA (noble gases) does not form anions full "s" and "p" subshells!







