9 • Orbital Hybridization
advertisement

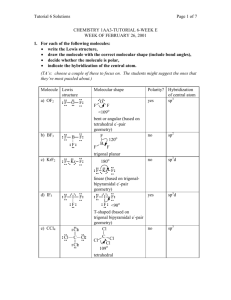
• AP Chemistry Name ____________________________________ 9 • Orbital Hybridization PRACTICE TEST 1. Which hybridization is associated with a steric 8. number of 3? d) sp3d a) sp b) sp 2 How many sigma () and pi () electrons pairs are in a carbon dioxide molecule? a) four and zero d) two and four 3 2 e) sp d b) three and two e) one and three c) sp3 c) two and two 2. The molecule BrF3 has a steric number of ___ on 9. the central atom? a) 3 3. b) 4 c) 5 d) 6 What is the hybridization of Br in BrF3? a) sp d) sp3d b) sp2 e) sp3d2 What is the hybridization of the oxygen atoms in CH3OH and CO2, respectively? a) sp3, sp3 d) sp2, sp2 b) sp3, sp2 e) sp3, sp c) sp2, sp3 10. All of the following species contain two -bonds c) sp3 EXCEPT 4. How many equivalent a) 3 b) 1 sp3d orbitals are there? c) 5 d) 6 a) SCN d) OCS b) CO e) NO c) H2CCO 5. What shape for electron pairs is associated with sp3d2 hybridization? 11. Which response contains all the characteristics that a) linear d) tetrahedral b) square planar e) octahedral should apply to BF3? 1. trigonal planar c) bent 2. one unshared pair of electrons on B 3. sp2 hybridized boron atom 6. A double bond contains ___ sigma bond(s) 4. polar molecule and ___ pi bond(s). 5. polar bonds a) 0, 2 7. b) 1, 2 c) 2, 0 d) 1, 1 Which of the following elements is most likely to 3 display sp d hybridization? a) oxygen d) carbon b) nitrogen e) boron c) phosphorus a) 2, 4, and 5 d) 1, 3, and 5 b) 1, 3, and 4 e) 3, 4, and 5 c) 1, 2, and 3 15. According to the VSEPR model, the progressive 12. The respective hybridizations of nitrogen in NF3 and NH3 are increase in the bond angles in the series of molecules H2, NH3, and CH4, is best accounted for by the sp2 and sp2 a) 2 b) sp and sp c) sp and sp3 3 d) sp and sp a) increasing size of the central atom 2 b) decreasing electronegativity of the central atom 3 c) decreasing number of unshared pairs of electrons d) increasing repulsion between hydrogen 13. Molecule Bond atoms Bond length (pm) NH3 N-H 98 PH3 P-H 140 16. A table of atomic radii is given on the table below. Based on the information given on the table above, which statement best explains the P-H bond length is longer than N-H bond length? a) The P atom has a larger atomic radii making the columbic attractions weaker and the bond length longer. b) The P atom has a larger atomic radii making the columbic attractions stronger and the bond length longer. c) The P atom has a smaller atomic radii making the columbic attractions weaker and the bond length longer. d) The P atom has a smaller atomic radii making the columbic attractions stronger and the bond length longer. 14. How many and bonds are in C2H2 in which the two carbon atoms are adjacent and each carbon atom has one hydrogen atom attached to it? a) three bonds and two bonds b) four bonds and one bonds c) three bonds and one bonds d) one bonds and one bonds Which of the following covalent bonds between hydrogen and a nonmetal would have the lowest overall potential energy based on the stomic radius of the atoms? a) ammonia b) hydrogen fluoride c) methane d) water Short Answer: 12. Consider the structural formula for acetic acid, HC2H3O2 or CH3COOH. Indicate the type of hybridization used by each of the carbon and oxygen atoms. H O H C C H O H 13. Consider the structural formula for the acetate ion, C2H3O2– or CH3COO–. Indicate the hybridization used by each of the carbon and oxygen atoms. H O H C C H O 14. Briefly explain why carbon as graphite (a nonmetal) can conduct electricity? 15. When BF3 + NH3 BF3NH3, how does the hybridization of the boron atom change, if at all?