Molecular Geometry and Polarization
advertisement

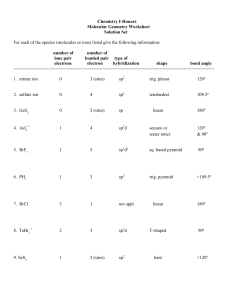
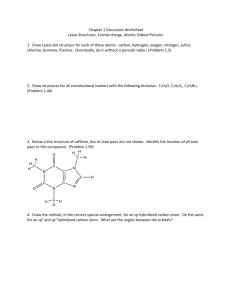
Molecular Geometry and Polarization Shapes of Molecules Valence Shell Electron Pair Repulsion Theory (VSEPR) a. Bonded electrons b. Lone Pairs 1. Linear (180o) BeH2 CO2 2. Trigonal Planar (120o) NO3- 3. Tetrahedral (109.5o) CH4 4. Trigonal Pyramidal (~107o) NH3 5. Bent (~104.5o) H2O H O H SO2 6. Trigonal Bipyramid (120o, 90o) PCl5 7. Octahedral (90o) SF6 Shapes of Molecules Ex: Multiple Bonds: N2 H2CO HCN SO2 Shapes of Molecules Models Activity SO22+ SO22SO2 SO3 SF3PF4XeCl5+ BrF4- Predict the molecular geometry of: SnCl3O3 SeCl2 CO32SF4 IF5 ClF3 ICl4- WarmUp ClF4SiCl3SO2 SCl4 SeO3 BrCl5 BrCl3 Polar Molecules 1. Polar molecule – Overall, the electrons are attracted more to one end of an entire molecule 2. Non-Polar Molecule – The electrons are spread out evenly over the entire molecule -/ + Partial (not full) charges Examples: H2 CH4 H2O H2CO Electron Density H2 H2O CH4 H2CO Polar Molecules BeCl2 NH3 CO2 SO2 SF6 BCl3 CH2Cl2 SCO CH3F BH2Cl PH3 CHF3 CH2F2 SO3 SO32- NF3 CH3CHO Hybrid Orbitals • A mixing of the atomic orbitals (s, p, d, f) of the central atom • Electrons no longer move in the old orbitals, but in a new pattern BeF2 Isolated Be 1s22s2 (Note that all Be: electrons are paired) To bond Be must unpair some electrons: Bonded Be 1s22s12p1 •Be• • Be is called an “sp” hybrid. • Drawings: Isolated Be BeF2 CH4 Isolated C 1s22s22p2 Bonded C 1s22s12p3 Isolated C Bonded C sp3 Effect of Lone Pairs • Lone pairs do count towards hybridization • Ex: H2O Try BF3 Examples CCl4 NH3 PF5 SF6 XeF4 BrF3 PH3 H2S SF5SF4 CO32HCN BrCl3 CH4 H2S SO2 SO22AsCl5 ClF3 KrF4 Hybrid Orbitals and Multiple Bonds • sigma () bonds – single bonds formed by hybrid orbitals • pi () bonds – double or triple bonds, not formed by hybrid orbitals H H–H C=C H One bond H :N=N: H One bond plus one bond One bond plus two bonds • Consider C2H4 • Each C is sp2 • Double bond does not count toward hybridization • Consider C2H2 • Each C is sp hybridized • Twobonds do not count toward hybridization What is the hybridization and bonding types for H2CO? Also, what are the bond angles? What is the hybridization and bonding types for acetonitrile (shown)? Also, what are the bond angles? H H - C -C=N: H Delocalized Bonding • Adjacent multiple bonds can overlap. • Benzene (C6H6) • All bond lengths are equal Use hybrid orbital theory to explain why all the bonds in the NO3- ion are of equal length 12 a) ~110o b) BF3 flat (no lone pair) 21. a) (lin)lin b) (tetr)tr. Py c) (Trig bi)ss d) (oh)oh e) (tetr)tetr f) (lin)lin 22 a) (Tetra) Trig. Pyramid b) (Trig planar), Trig pl c) (Tr. Bipy) T d) (Tetra) Tetra e) (Trig Bipy) lin f) (Tetra) Bent 24 a) i) Octa (sq.planar) ii) Tetrahedral iii) Trig Bipyr.(see-saw) b) i) Two ii) O iii) One c) S or Se d) Xe 26. a) 104.5o, 120o b) 109.5o, 120o c) 107o, 104.5o d) 180o, 109.5o 28. 2 LP (NH2-, ~109o), 1 LP (NH3, 107o), 0 LP (NH4+, 109o) 30. a) ClO2- (~109.5o, 2LP) NO2- (120o, 1 LP) b) XeF2 (4 LP around the center) 32. a) Lone Pair on P b) Lone Pair on center O 36. Polar = (b), (c), (e) 38.Ortho and meta 44. Not enough p suborbitals 46. SF2 = sp3, SF4 = sp3d 48. a) sp3 b) sp c) sp2 d) sp3d e) sp3d2 52. b) N2H4 (sp3), N2 (sp) c) N2 stronger bond 54. a) sp3 (C-H), sp2 (C-O) b) 36 ve c) 26 ved) 2 ve- in double e) 8 ve- in lone pairs 56. a) 1, 120o 2, 120o 3, 105o b) sp2, sp2, sp3 c) 21 bonds 62. 100. In2S (I) [Kr]5s24d10 InS (II) [Kr]5s14d10 In2S3 (III) [Kr]4d10 In(III) is smallest (least mutual electron repul) In(III) has the highest lattice energy 102.a) C2H3Cl3O2 b) C2H3Cl3O2 c) Structure CCl CH(OH)