Answers
advertisement

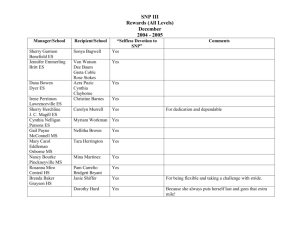
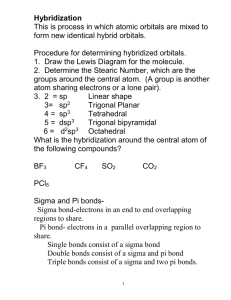
Tutorial 6 Solutions Page 1 of 7 CHEMISTRY 1AA3-TUTORIAL 6-WEEK E WEEK OF FEBRUARY 26, 2001 1. For each of the following molecules: • write the Lewis structure, • draw the molecule with the correct molecular shape (include bond angles), • decide whether the molecule is polar, • indicate the hybridization of the central atom. (TA’s: choose a couple of these to focus on. The students might suggest the ones that they’re most puzzled about.) Molecule Lewis structure a) OF2 F O F b) BF3 F B F F c) KrF2 Molecular shape F O F <109o bent or angular (based on tetrahedral e--pair geometry) F 120o B F F trigonal planar 180o F Kr F Polarity? Hybridization of central atom yes sp3 no sp2 no sp3d yes sp3d F Kr F linear (based on trigonalbipyramidal e--pair geometry) d) IF3 F I F C Cl F F Cl Cl I <90o T-shaped (based on trigonal bipyramidal e--pair geometry) no Cl F e) CCl4 F Cl C Cl Cl 109o tetrahedral Cl sp3 Tutorial 6 Solutions Page 2 of 7 f) SO2 O S O g) SO3 O S O O O S O <120o bent or angular (based on trigonal-planar e--pair geometry) O O S o yes sp2 no sp2 yes sp3 yes sp3d no sp3d2 O 120 trigonal planar h) SF2 F i) SF4 S F F F S F F j) SF6 F F F S F F F F S F <109o bent or angular (based on tetrahedral e--pair geometry) F o S F <120 F F <90o seesaw (based on trigonalbipyramidal e--pair geometry) F F S F F F F 90o octahedral 2. One of the first drugs to be approved for use in the treatment of AIDS is azidothymidine (AZT). Complete the Lewis structure of AZT, shown below, by adding the missing lone pairs of electrons and any nonzero formal charges. The structure has been modified accordingly. Lone pairs were added to give the atoms (other than H) complete octets. Then formal charges were calculated. Note that for the atoms with no formal charge, all C’s have 4 bonds, all N’s have 3 bonds, and all O’s have 2. This is as you would predict from the number of valence electrons needed in order to complete the octets. This is a handy way of viewing Lewis structures, and allows you to draw complex structures more easily than the routine we learned in Chem 1A03. Note, however, that this applies ONLY to atoms with no formal charge! Tutorial 6 Solutions Page 3 of 7 H H O H O N C O H C C N C C H H H C O H C H H C H C C H N H N N a) How many carbon atoms are sp3 hybridized? All of the tetrahedral C’s (all the C’s with 4 single bonds) are sp3 hybridized. There are 6 of them (i.e. all of the C’s EXCEPT the ones in the six-membered ring). b) How many carbon atoms are sp2 hybridized? The C atoms in the six-membered ring are all triangular (trigonal) planar (they are all surrounded by three electron-pair domains). Therefore these 4 C atoms are sp2 hybridized. c) Which atom is sp hybridized? The middle N of the –N3 group has only two electron-pair domains (i.e. linear geometry), and is therefore sp hybridized. d) How many σ bonds are in the molecule? The framework of the molecule is made of σ bonds. That is, there is a σ bond between every pair of bonded atoms. Therefore, the total number of σ bonds is 33. e) How many π bonds are in the molecule? When you see a multiple bond, the “first” bond is a σ bond, and any additional bonds must be π bonds. Therefore, there are 5. f) What is the N-N-N bond angle in the azide (-N3) group? The central N has two electron-pair domains, so the geometry around it must be linear. That is, the angle is 180°. g) What is the H-O-C bond angle in the side group attached to the fivemembered ring? The O has four electron-pair domains, so the geometry around it is based on a tetrahedron (actually, it’s bent). The angle is slightly less than 109°. Remember that Lewis structures show only which atoms are connected to which, not the correct geometry! h) What is the hybridization of the oxygen atom in the –CH2OH group? The O has four electron-pair domains, so it’s sp3 hybridized. Tutorial 6 Solutions Page 4 of 7 3. For the following molecules, draw a complete orbital picture to show the bonding involved. Your picture should clearly show the 3-D shape of the molecule. Clearly indicate the types of orbitals involved (s, p, sp, sp2, sp3, etc.) and the types of bonds formed. a) H2CO H C O H Note that both the C and the O are surrounded by three electron-pair domains, and so they are sp2-hybridized. Therefore, the structure can be built by combining two sp2hybrid building blocks (see p. 389 in your text): sp2 sp2 p sp 2 sp2 p sp2 sp2 sp2-hybridized atom sp2-hybridized atom Now bring these “puzzle pieces” together, and add the H atoms (let the H 1s orbitals interact with the “unbonded” sp2 orbitals on the C). Note the lone pairs in two of the sp2-hybrid orbitals on the O: p s 2 ss sp p 2 sp sp 2 sp2 Note that all of the end-to-end overlaps produce σ bonds; there is also one π bond formed by side-to-side overlap of p orbitals. b) HCN H C N Note that both the C and the N are surrounded by two electron-pair domains, and so they are sp-hybridized. Therefore, the structure can be built by combining two sphybrid building blocks (see p. 389 in your text): sp p p sp sp-hybridized atom sp p p sp sp-hybridized atom Now bring these “puzzle pieces” together, and add the H atom (let the H 1s orbital interact with the “unbonded” sp orbital on the C). Note the lone pair in the sp-hybrid orbital on the N: Tutorial 6 Solutions Page 5 of 7 s sp p p sp spp p sp Note that all of the end-to-end overlaps produce σ bonds; there are also two π bonds formed by side-to-side overlap of p orbitals. c) allene, CH2=C=CH2 H H C C C H H Note that the two C’s on the ends are surrounded by three electron-pair domains, and so they are sp2-hybridized. The central C has only two electron-pair domains, and is sp-hybridized. Therefore, the structure of allene can be built by combining the following building blocks (see p. 389 in your text): sp2 sp2 p sp sp p 2 sp2-hybridized atom p sp sp-hybridized atom sp 2 p sp2 sp2 sp2-hybridized atom Now let’s start building the carbon chain, one pair at a time: sp2 sp2 p sp sp p 2 p sp sp 2 p sp2 sp2 Note that the two atoms were brought together such that the p orbitals were lined up side by side, allowing a π bond to form. This is important to note before we bring in the remaining C. We see that the “unbonded” p orbital remaining on the sp-hybridized atom is perpendicular to the page. Therefore, if we want to make another π bond, we’ll have to rotate the other atom so that its p orbital is also pointing out of the page (otherwise, the two p orbitals couldn’t line up with each other): sp2 sp2 p sp2 sp p p sp sp2 p sp2 sp2 Now bring these “puzzle pieces” together, and add the H atoms (let the H 1s orbitals interact with the “unbonded” sp2 orbitals): Tutorial 6 Solutions p s 2 s sp sp sp p 2 Page 6 of 7 p sp sp2 p sp2 s sp2 s Note that all of the end-to-end overlaps produce σ bonds; there are also two π bonds formed by side-to-side overlap of p orbitals. Finally, for allene, use your picture to decide whether the four hydrogen atoms are in the same plane. If not, what is their spatial relationship? From this picture, we can see that the H’s are NOT all in the same plane. The plane that contains the H’s on one end is perpendicular to the plane that contains the H’s on the other end. TA’s: I’ll bring a molecular model to the tutorial rooms; it should really help the students “see” the solution. 4. Draw as many structural isomers of C5H10 as you can. Can you find more than I could? Be careful not to include any duplicates! Note that this formula CANNOT represent an acyclic alkane: there aren’t enough H’s to satisfy the general alkane formula, CnH2n+2. This is CnH2n: two H’s are “missing”! So all isomers of this formula must have a double bond or a ring. It’s a good idea to be systematic, so that you don’t miss any isomers! Start with chains of 5 C’s, then try 4 C’s, etc. Don’t forget the rings! And finally, be careful to show the correct number of H’s on each C to bring the total number of bonds to 4. CH3 CH2=CHCH2CH2CH3 CH3CH=CHCH2CH3 CH3 CH3 CH3 CH3 CH2=CCH2CH3 CH3 CH3C=CHCH3 CH3 CH3CHCH=CH2 So I found a total of 10! CH3 CH2CH3 Tutorial 6 Solutions 5. Page 7 of 7 Arrange the following sets of compounds in order of increasing boiling point (and, as always, be prepared to justify your answer!). a) CH3CH2CH2CH2CH3 < CH3CH2CH2CH2Cl < CH3CH2CH2CH2OH The compound with the weakest intermolecular forces will have the lowest bp, because for that compound, the least energy will be required to overcome the IMF’s. Pentane will have the lowest bp. Because it is nonpolar (C-H bonds have a very small difference in electronegativities), the only forces attracting one molecule to another are dispersion forces (the weakest type of IMF). 1-Chlorobutane will have the intermediate bp. The C-Cl bond is slightly more polar than C-H bonds, so dipole-dipole interactions will be greater than in pentane. And probably more importantly, since Cl is a larger, more polarizable atom than C, the dispersion forces in 1-chlorobutane will be stronger than the dispersion forces in butane. Finally, 1-butanol will have the highest bp; because of the –OH group, it is capable of hydrogen bonding. (Remember that “hydrogen bonds” are NOT covalent bonds; they are simply very strong dipole-dipole interactions between different molecules.) b) < < Since all of these molecules are nonpolar, we must be comparing the strengths of the dispersion forces in them. C5H12 will definitely have the highest bp, because dispersion forces are stronger when there are more electrons in the molecule (bigger molecules). Of the two isomers of C4H10, 2-methylpropane will have the lower bp, because it has a smaller surface area for interaction with other molecules. More compact alkanes will always have lower boiling points (weaker IMF’s) than their straight-chain isomers.