Key
advertisement
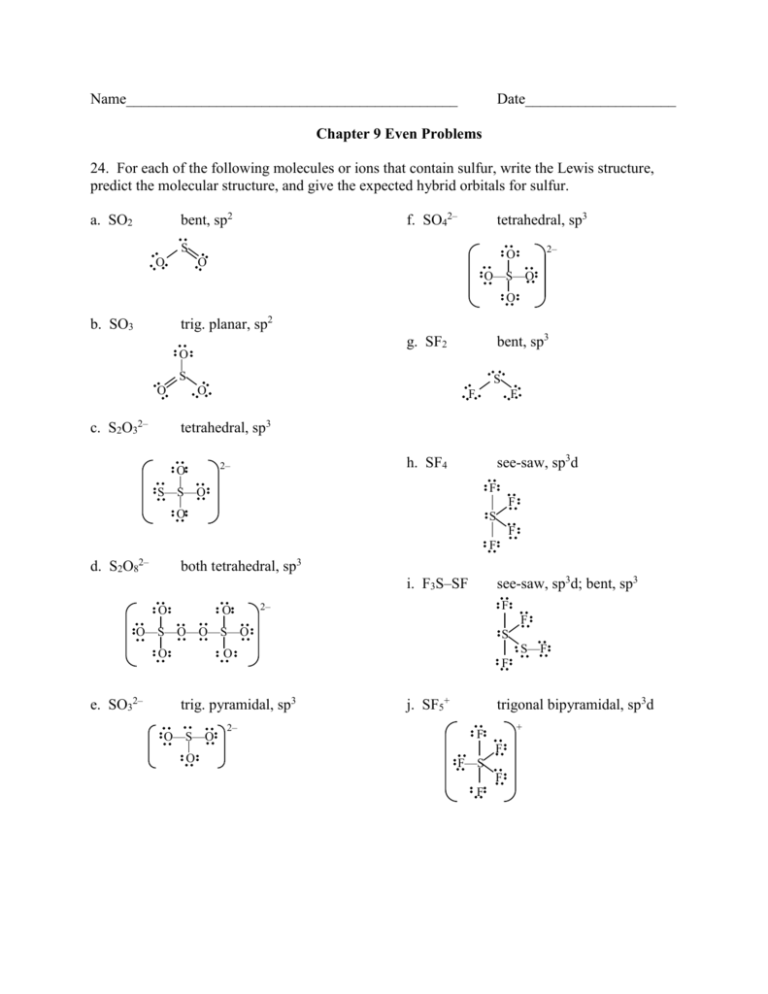
Name____________________________________________ Date____________________ Chapter 9 Even Problems 24. For each of the following molecules or ions that contain sulfur, write the Lewis structure, predict the molecular structure, and give the expected hybrid orbitals for sulfur. bent, sp2 a. SO2 f. SO42– tetrahedral, sp3 S O 2– O O O—S—O O trig. planar, sp2 b. SO3 bent, sp3 g. SF2 O S O c. S2O32– S O F F tetrahedral, sp3 see-saw, sp3d h. SF4 2– O S—S—O F O S F F F d. S2O82– both tetrahedral, sp3 see-saw, sp3d; bent, sp3 i. F3S–SF O O F 2– F O—S—O—O—S—O O e. SO32– S S—F O trig. pyramidal, sp3 O—S—O O 2– F j. SF5+ trigonal bipyramidal, sp3d + F F F—S F F 28. Many important compounds in the chemical industry are derivatives of ethylene, C2H4. Two of them are acrylonitrile (the first one) and methyl methacrylate. H H H C=C H C H N sp2 sp2 CH3 C=C sp2 sp C–O–CH3 O sp2 sp3 sp2 sp3 Complete the Lewis structures, showing all lone pairs. Give the hybridization of all carbon atoms. In acrylonitrile, how many of the atoms in the molecule lie in the same plane? How many bonds and how many bonds are there in each compound? acrylonitrile: all atoms in the same plane; 6 bonds, 3 bonds methyl methacrylate: 14 bonds, 2 bonds 32. The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in Tanzania. The structure for thiarubin-A is H H C–C H3C–C≡C–C C–C≡C–C≡C–CH=CH2 S—S a. Complete the Lewis structure showing all lone pairs of electrons. b. Indicate the hybrid orbitals used by the carbon and sulfur atoms. sp3–sp–sp–sp2 sp2–sp2 sp2–sp–sp–sp–sp–sp2–sp2 sp3–sp3 c. How many and bonds are present in this molecule? 23 , 9 34. Which of the following are predicted by the molecular orbital model to be stable diatomic species? a. N22–, O22–, F22– draw MO diagrams: N22– bond order = (6–2)/2 = 2 stable O22– bond order = (6–4)/2 = 1 stable F22– bond order = (6–6)/2 = 0 unstable b. Be2, B2, Ne2 Be2 bond order = (2–2)/2 = 0 unstable B2 bond order = (2–0)/2 = 1 stable Ne2 bond order = (6–6)/2 = 0 unstable 40. Construct a molecular orbital energy-level diagram for the Cl2 molecule. Is Cl2 expected to be paramagnetic? ___ 3p* 3p ↑↓_ ↑↓_ ↑__ ↑↓_ ↑↓_ 3p* ↑↓_ ↑↓_↑_ 3p ↑↓_ ↑↓_ 3p ↑↓_3p 3s* ↑↓_ 3s ↑↓_3s ↑↓_ 3s ↑↓_ bond order = (8–6)/2 = 1 diamagnetic 61. (challenge problem) Two structures can be drawn for cyanuric acid: H O N C N H O C H N O–C N C N H O O–H C N C O H Complete the Lewis dot structures above (add lone pairs). a. Are these two structures the same molecule? Explain. They are both H3C3N3O3, but their atoms are connected differently: they are isomers. b. Give the hybridization of the carbon and nitrogen atoms in each structure. structure 1: carbons are sp2, nitrogens are sp3 structure 2: all carbons and nitrogens are sp2 c. Use bond energies to predict which form is more stable; that is, which contains the strongest bonds? structure 1: 3 (C=O) + 6 (C–N) + 3 (N–H) 3 (745 kJ) + 6 (305 kJ) + 3 (391 kJ) = 5238 kJ structure 2: 3 (C–O) + 3 (C–N) + 3 (C=N) + 3 (O–H) 3 (358 kJ) + 3 (305 kJ) + 3 (615 kJ) + 3 (467 kJ) = 5235 kJ Structure 1 has slightly stronger bonds, but the two structures are essentially comparable.