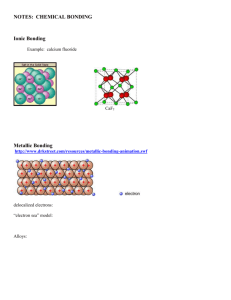
Identifying types of IMF`s – intermolecular forces
advertisement
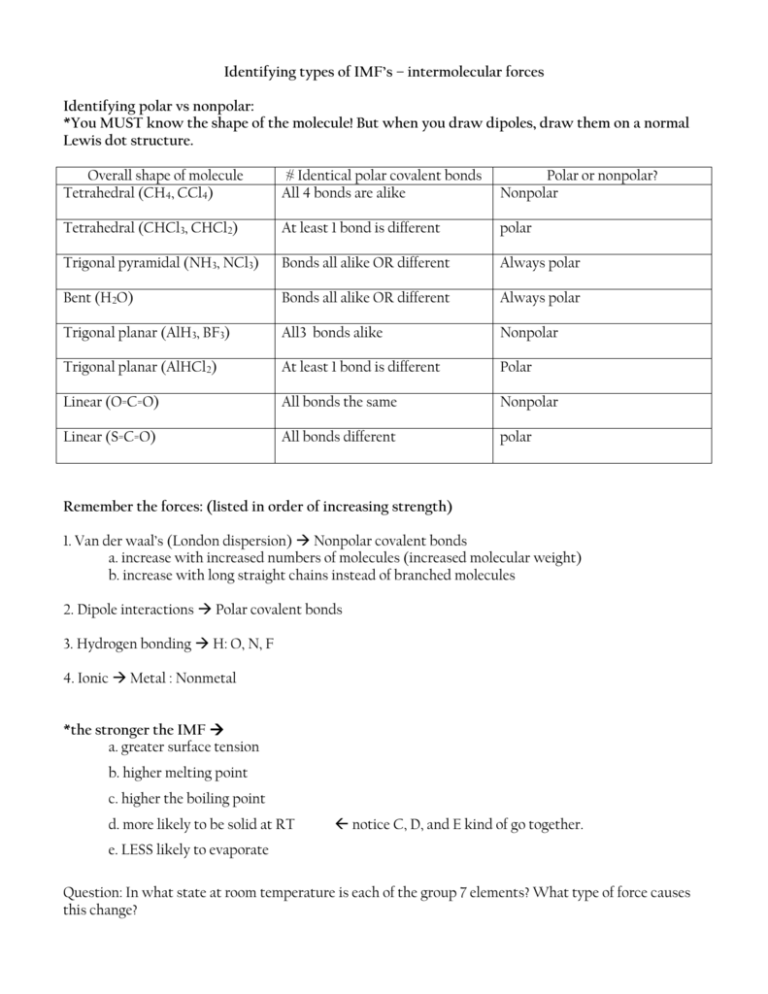
Identifying types of IMF’s – intermolecular forces Identifying polar vs nonpolar: *You MUST know the shape of the molecule! But when you draw dipoles, draw them on a normal Lewis dot structure. Overall shape of molecule Tetrahedral (CH4, CCl4) # Identical polar covalent bonds All 4 bonds are alike Polar or nonpolar? Nonpolar Tetrahedral (CHCl3, CHCl2) At least 1 bond is different polar Trigonal pyramidal (NH3, NCl3) Bonds all alike OR different Always polar Bent (H2O) Bonds all alike OR different Always polar Trigonal planar (AlH3, BF3) All3 bonds alike Nonpolar Trigonal planar (AlHCl2) At least 1 bond is different Polar Linear (O=C=O) All bonds the same Nonpolar Linear (S=C=O) All bonds different polar Remember the forces: (listed in order of increasing strength) 1. Van der waal’s (London dispersion) Nonpolar covalent bonds a. increase with increased numbers of molecules (increased molecular weight) b. increase with long straight chains instead of branched molecules 2. Dipole interactions Polar covalent bonds 3. Hydrogen bonding H: O, N, F 4. Ionic Metal : Nonmetal *the stronger the IMF a. greater surface tension b. higher melting point c. higher the boiling point d. more likely to be solid at RT notice C, D, and E kind of go together. e. LESS likely to evaporate Question: In what state at room temperature is each of the group 7 elements? What type of force causes this change?