Lecture 8 - The Spectrochemical Series – Color and Magnetism 1
advertisement
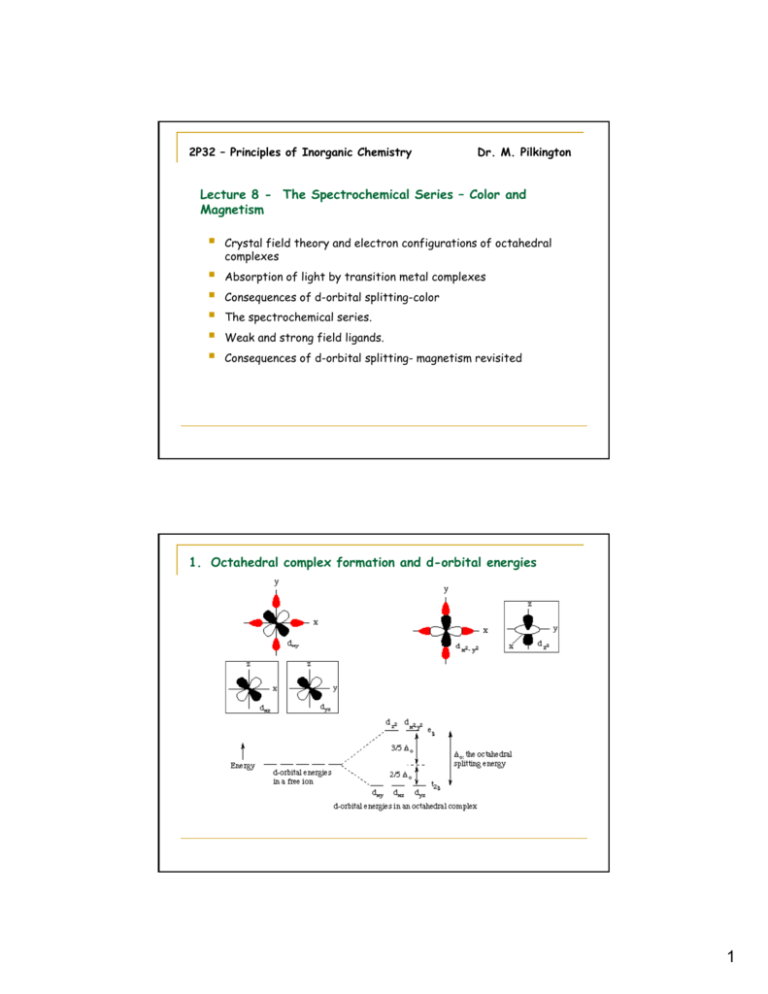
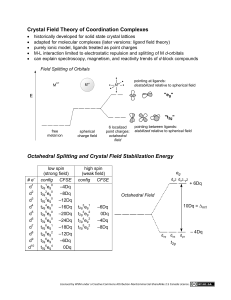
2P32 – Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 8 - The Spectrochemical Series – Color and Magnetism Crystal field theory and electron configurations of octahedral complexes Absorption of light by transition metal complexes Consequences of d-orbital splitting-color The spectrochemical series. Weak and strong field ligands. Consequences of d-orbital splitting- magnetism revisited 1. Octahedral complex formation and d-orbital energies 1 Electronic configuration of the d-orbitals 2. Absorption of Light by Transition Metal Complexes. If a compound is colored, it must absorb visible light. To absorb visible light a compound must have an empty (or partially filled) electronic energy level that is just a little higher in energy than another filled (or partially filled) level. The d orbitals in transition-metal ions often meet this test, remember back to the colors of the different structural isomers. 2 Effects of ligands on the colors of coordination compounds The color wheel This color wheel demonstrates which color a compound d will ill appear if iit only l h has one absorption in the visible spectrum. For example, if the compound absorbs red light, it will appear green. 3. Consequences of d-Orbital Splitting: Color Color in transition metal complexes is due to an electron being excited from one d-orbital to a higher-energy d-orbital. In the case of octahedral complexes, an electron is moved from a t2g orbital to an eg orbital. The energy difference for the first transition series generally falls in the visible region. Absorption of one color in the visible spectrum results in the ion having the complementary color. The amount of d-orbital splitting depends on the ligands, thus different ligands have different splitting energies (values of o), and different colors result. 3 Illustration of Crystal Field Theory – Example 1 [Ti(H2O)6]3+is a d1 complex and the electron occupies the lowest energy orbital available, i.e. one of the three degenerate t2g orbitals. The purple color is the result of the absorption of light which results in the promotion of this t2g electron into the eg level. t2g1eg0 g g –> t2g0eg1 g g The UV-Vis absorption spectrum reveals that this transition occurs with a maximum at 20300 cm-1 which corresponds to o 243 kJ/mol. (1000 cm-1 = 11.96 kJ/mol , 2.86 kcal/mol or 0.124 eV.) Typical o values are of the same order of magnitude as the energy of a chemical bond. It is common in transition metal chemistry to report the crystal field splitting energy o, the pairing energy P, and even the absorption spectra in terms of the wavenumber (cm-1) rather than the wavelength. The wavenumber is the number of waves in a given length. The relationships between the frequency, the wavelength, the wavenumber and the energy are as follows: wavenumber, 4 Example 2 Now we will look at the absorption spectra of two cobalt(II) species. Crystalline cobalt(II) chloride contains octahedral cobalt(II) surrounded by six chloride ions. It is deep blue in color, because the cobalt absorbs light in the yellow part of the spectrum. On the other hand, the hexaaquocobalt(II) cation absorbs light in the green part of the spectrum, resulting in a purplish pink color. o = 206 kJ/mol o = 232 kJ/mol The reason that the two species are differently colored is because the d-orbital splitting energy is different when chloride is a ligand than when water is a ligand. Remember that the reason these two high-spin cobalt species are colored is because the energy separation between the t2g and the eg levels in the cobalt (II) ion lies in the visible region of the spectrum. Absorption of light occurs because an electron can be excited from a lower energy level (a t2g orbital in this case) to a higher energy orbital (eg in this case). 5 4. The Spectrochemical Series It is possible to arrange ligands into a series that reflects their ability to split the d-orbitals. This series is as follows (very important to learn): I- < Br- < Cl- < SCN- < NO3- < F- < OH- < H2O < NCS- < gly < py < NH3 < en < NO2- < PPh3 < CN- < CO Magnitudes of Crystal Field Splitting Energies o (kJ/mol) There is can be a factor of 2 difference between the weakest and the strongest ligands. This explains why coordination complexes of the same metal with the same oxidation state but different ligands vary so much in color. There are also variations in the octahedral splitting energy with changing oxidation state of the metal and changing period number. This explains why when the oxidation number is changed the color of the complex changes. 6 5. Weak and Strong Field Ligands - Summary Large splitting is associated with strong field ligands. Small splitting is associated with weak field ligands. Thus, ammine ligands split the d-orbitals more than the fluoride ligands for the Cobalt(III) ion as shown below: 7 I- < Br- < Cl- < SCN- < NO3- < F- < OH- < H2O < NCS- < gly < py < NH3 < en < NO2- < PPh3 < CN- < CO 8 When o < pairing energy P – electron goes into the eg orbital – HIGH SPIN COMPLEX When o> pairing energy P then electron goes into the t2g orbital – LOW SPIN COMPLEX The value of the splitting energy o depends on the ligands position in the spectrochemical series. If the ligand is a weak field splitter then the complex will be high spin. If th the li ligand d iis a strong t fi field ld splitter litt th then th the complex l will ill b be llow spin. 9 6. Revisiting Crystal Field Theory and Magnetism The Gouy balance is used to measure magnetic susceptibility M From this we determine the effective magnetic moment and number of unpaired electrons for a transition metal complex. By y knowing g if f a particular p ligand g is a strong g or weak splitter p and by y measuring the magnetic susceptibility of a transition metal complex we can rationalize how to fill the d-orbitals and assign the complex as being High or Low spin. 10