The Synthesis of trans-Dichlorobis(ethylenediamine)cobalt(III
advertisement

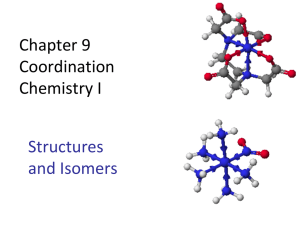
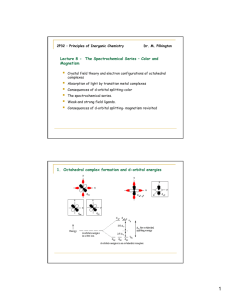
The Synthesis of trans-Dichlorobis(ethylenediamine)cobalt(III) Chloride • To learn about Coordination Compounds and Complex Ions. • To learn about Isomerism. • To learn about the Color of Transition Metal Complexes. In this laboratory exercise, we will synthesize a Coordination Compound of Cobalt, transDichlorobis(ethylenediamine)cobalt(III) Chloride, [Co(en)2Cl2]Cl, from Cobalt(II) Chloride Hexahydrate, CoCl2•6H2O. 4CoCl2•6H2O+ 8en + 4HCl +2H2O2 4[Co(en)2Cl2]Cl + 28H2O (Eq. 1) "en" is short-hand for Ethylenediamine: NH2-CH2CH2-NH2 This synthesis involves oxidation of the Cobalt from Co+2 to Co+3 by hydrogen peroxide: 2 Co2+ + H2O2 + 2 H+ 2 Co3+ + 2 H2O (Eq. 2) (Here, H2O2 acts as an Oxidizing Agent and is reduced from O-1 to O2-.) However, Co3+ is unstable in an aqueous environment, being readily reduced back to Co2+. To prevent this from happening, the resulting Co3+ can be stabilized by adding an Ethylenediamine ligand. Hence, if the oxidation is carried out in the presence of Ethylenediamine, the Co3+ formed is stabilized and retains the higher oxidation state. [Co(en)2Cl2]Cl is a Coordination Compound. Coordination Compounds are substances that contain at least one Complex Ion, a species containing a central metal cation that is bonded to molecules or anions, called Ligands. In the present case, the coordination compound [Co(en)2Cl2]Cl is formed from the Co(en)2Cl2 + complex cation and the Cl- anion. The Complex itself contains four Ligands; two Cl-‘s and two Ethlyenediamines (en). (Note the [ ]’s around the Complex Ion Co(en)2Cl2+ in the chemical formula.) Like other Water soluble compounds, this ionic substance can dissolve in Water according to: [Co(en)2Cl2]Cl(aq) Co(en)2Cl2 + (aq) + Cl-(aq) (Eq. 3) The covalent bonding between the metal ion and its ligands, in our case, between the Co3+ and the Cl-‘s and en’s, is via Coordinate Covalent Bonds; the ligands act as Lewis Bases and donate pairs of electrons to the metal, acting as a Lewis Acid. As a general example of this type of bonding, consider the formation of the Co(NH3)63+ complex ion. The NH3 ligand has one lone pair of electrons which can coordinate covalent bond to the Co3+ metal center: Since this example complex contains six NH3 ligands, it has six coordinate covalent bonds arranged octahedrally about the metal center: The number of coordinate covalent bonds formed is referred to as the Coordination Number of the complex. In the above example, the coordination number is six. The coordination number determines the geometry of the complex. Typical coordination geometries are: Coordination Number 2 4 6 Geometry Linear Square Planar or Tetrahedral Octahedral For our complex ion, Co(en)2Cl2+, the coordination number is also six. 2 electron pairs are donated to the Co3+ ion by each chloride ion, Cl-, ligand. The remaining 4 electron pairs are donated by the two Ethylenediamine ligands, the Ethylenediamine ligand being bidentate; forming two bonds to the metal per “en”. Because each “en” ligand in our Complex is bidentate, two different Isomers of our Complex are possible; a cis form and a trans form. These are pictured below: We will be synthesizing the trans isomer (Green) of this complex, although some of the cis isomer (Purple) will be formed as an impurity. Isomers are substances that have the same chemical formula, but are different compounds; i.e., each has its own set of physical and chemical properties. Each of the above pictured isomers has the chemical formula CoCl2C4H16N4 + and yet the trans form is distinctly different than the cis form, as can be seen from the fact that the former is a Green color and the latter is a Purple color. A more obvious example is the isomerism exhibited by compounds with the chemical formula C2H6O. Two isomers with this formula are: Dimethyl Ether CH3-O-CH3 Ethanol CH3CH2-OH These two Structural Isomers have the same chemical formula C2H6O, but the bonding between the atoms is distinctly different. (In Dimethyl Ether the Oxygen is bound to two Carbons; C-O-C. In Ethanol, the Oxygen is bound to a Carbon and a Hydrogen; C-O-H.) These bonding differences manifest themselves in differences in the Physical and Chemical Properties of the two substances. Dimethyl Ether Ethanol Boiling Pt. -23oC Boiling Pt. 78.4oC Melting Pt. -138.5oC Melting Pt. -114.3oC Density 1.59 g/L Density 0.789 g/mL Transition metal complex ions exhibit a number of different types of isomerism: Coordination Isomers The composition of the complex ion changes, but not that of the compound. Ex: Compounds with the formula CoCrC6H18N12 Cmpd 1: [Co(NH3)6][Cr(CN)6] Cmpd 2: [Cr(NH3)6][Co(CN)6] Note the metal center of each complex has switched. Linkage Isomers The composition of the complex ion remains the same, but the binding attachment of the ligand donor atom changes. Ex: Complexes with the formula CoN6H15O22+ Cmpl 1: Co(NH3)5ONO2+ Cmpl 2: Co(NH3)5NO22+ Note that in Complex 1 the bonding to the NO2- ligand is through the O atom, whereas in Complex 2 it is through the N atom. Geometric Isomers Atoms or groups of atoms are arranged differently in space relative to the central atom. (cis-trans isomerism is an example of this type of isomerism.) Ex: The cis-trans isomers of our Cobalt complex are example of this type of isomerism. Finally, it will be noted the starting material, Cobalt (II) chloride hexahydrate, is a brightly colored pink-purple substance, whereas our product is a deep forest green. This bright coloring is fairly common for transition metal complexes. Electrons in partially filled d-orbitals of a transition metal center, a distinctive feature of the transition metals, can absorb light of visible wavelengths. The color absorbed is then the complement of the color we see. The absorption of relatively low energy visible light occurs because of splitting of the d-orbitals’ energy levels. This is due to an interaction between the donated electron pair of the ligand with the lobes of the d-orbitals on the metal center; with the splitting pattern determined by the geometry of the ligands about the d-orbitals. Recall, the d-orbitals have the following shapes: So, if the negatively charged bonding electron pairs of the ligands interact with high electron density regions of say the dx2-y2 orbital along the x and y axes: the interactions are unfavorable and the energy of the system rises. If, on the other hand, the electron pairs of the ligands approach a dxy orbital along these same axes, the approach is along a nodal plane and the interaction is favorable, causing the energy of the system to drop. Thus, if the ligands are arranged octahedrally about the metal center, the d-orbital energies will split according to the following pattern: The Co(en)2Cl2+ complex ion we are synthesizing has a Co3+ center with an electron configuration of: Co3+ = [Ar] 3d6 In the presence of octahedrally arranged ligands, the five 3d orbitals will split according to: When a photon of Red light impinges upon the complex, its energy will just match the splitting energy and it will be absorbed. This will result in a Green colored compound. ∆E = Ephoton = hc/λ (Eq. 4) Here, h is Planck’s Constant, c is the Speed of Light and l is the wavelength of the photon absorbed. The magnitude of the splitting for a given complex is determined by the strength of the interaction between the ligands and the d-orbitals, the geometry of the ligands and the number of electrons occupying the d-orbitals. Thus, we will be synthesizing a particular isomer (trans) of the coordination compound [Co(en)2Cl2]Cl where the complex ion Co(en)2Cl2+ adopts an octahedral geometry due to the six coordinate covalent bonds formed with the ligands. Further, this complex is Green because of the particular splitting of the d-orbitals of the Co3+ center. Apparatus and Reagents: 250 mL beaker, casserole or large evaporating dish, graduated cylinder. Buchner funnel and suction filtration apparatus, cobalt(II) chloride hexahydrate, 30% hydrogen peroxide, concentrated hydrochloric acid, 10% ethylenediamene solution, 95% ethanol. Safety: % hydrogen peroxide can be corrosive and should be treated much the same as concentrated acid. Hydrochloric acid is corrosive Ethanol is flammable. To avoid skin contact with hydrogen peroxide and hydrochloric, you may wear gloves. Normally lightweight vinyl ves are provided for this se. These provide limited protection from incidental contact with the chemicals used If you suspect that your gloves have come in contact with a chemical, remove the gloves carefully to avoid contamination. Discard the gloves, wash your hands, and put on a new pair of gloves. Waste Disposal: All filtrates, washes, and excess reagents should be collected in a waste bottle. This waste is considered to be hazardous and should NOT be poured down a lab drain. Problems with chemical waste will be minimized if each student/pair removes ONLY the amount of reagent needed for the experiment. 5.0g cobalt(II) chloride hexahydrate 12 mL concentrated hydrochloric acid 2 mL 30% hydrogen peroxide 19 mL 10% ethylenediamene solution 15mL ethanol Procedure: 1. In a 250 mL beaker, dissolve 5.0g of CoCl2•6H2O in 12 mL of distilled water. To this solution add with stirring 19 mL of 10% ethylenediamene solution. 2. Slowly add 2 mL of 30% H2O2 while stirring the solution vigorously. 3. When the effervescence has almost ceased, slowly add with stirring 12 mL of 12M (concentrated) HCl. 4. Transfer the solution to a casserole or large evaporating dish. 5. Set the casserole on one of the hotplates in the hood. Heat the casserole, allowing the solution to evaporate until crystals form on the surface of the liquid. (This will probably require about 20-30 minutes) Continue to heat the solution for about five minutes after the entire surface is covered with crystals. 6. Cool the solution and crystals in an ice-water bath for about 20 minutes. 7. With a stirring rod, push any crystalline material that has collected on the casserole into the mixture. 8. Filter the mixture through a Buchner funnel. Wash the crystals with two 7 mL portions of 95% ethanol. 9. Examine your product. Only the green crystals should be present. If any white or colorless crystals are present, consult your instructor. Transfer the crystals to a watch glass and dry them in an oven for about 10 minutes at about 105°C. Allow the crystals to cool to room temperature before weighing them. 10. Weigh a clean dry glass vial. Transfer the crystals to the weighed vial. Reweigh the vial. Cap it and store for use in a future experiment. Calculations: 11. Calculate the number of moles of each reactant used (for solutions you want the moles of solute only). 12. Identify the limiting reactant. 13. Calculate the theoretical yield and the percent yield. Adapted from “The Synthesis of trans-Dichlorobis(ethylenediamine)cobalt(III) Chloride” (New Mexico Tech CHEM122 lab manual) and “The synthesis of a cobalt complex” (Murray State University CHE202 Lab Manual) Name_________________________________________ Mass of CoCl2•6H2O, g Volume of 30% H2O2 Volume of 10% C2H4(NH2)2, mL Volume of 12M HCl Mass of beaker plus product, g Mass of 50 mL beaker Mass of product Reactant Molar Mass (g/mol) Mass of reactant (for solution give mass of solute only) CoCl2•6H2O 30% H2O2 (density = 1.44 g/mL) C2H4(NH2)2 (density = 0.99 g/mL) HCl Moles of product expected Theoretical Yield, g Actual Yield, g % yield Moles of reactant Moles of product if ALL this reactant were used up