introduction of d-block elements 1-1-
advertisement

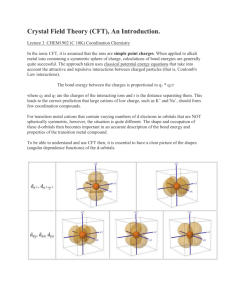
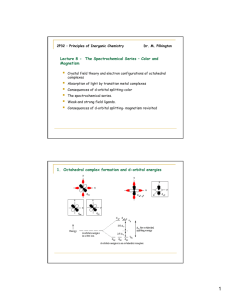
INTRODUCTION OF D-BLOCK ELEMENTS Why are they called d-block elements? Their last electron enters the d-orbital. Most d-block elements are also called transition metals. This is because they exhibit characteristics that ranges from Group 2 to Group 3 and have incomplete d- sub shell in any of their normal oxidation states. Zinc and Scandium are NOT considered as transition metals. Special properties of d- block elements 1. 2. 3. 4. Variable oxidation states Complex ion formation Colored compound formation Act as catalysts. Variable Oxidation States D-block elements exhibit variable oxidation states. This means that they can form two or more different types of cations. Examples: Iron can form both Fe²⁺ and Fe ³⁺ Manganese can Mn²⁺, Mn³⁺, Mn⁴⁺, Mn⁶⁺ and Mn⁷⁺ How come? The 3d energy level is very close to the 4s energy level. Very small difference in Ionisation energies. Electrons can therefore be removed from both the 4s and the 3d energy levels during ion formation. This leads to one element being able to form cations with more than one charge or valency. Complex ion Formation D- block elements are able to form complexes with ligands. This is because; they have incomplete d-orbital. they act as electron pair acceptors. What are ligands? Ligands are species with a lone pair of electrons. Ligands may be negatively charged F⁻, Cl⁻, CN⁻, etc Or neutral molecules H₂O,CO,NH₃ etc They donate their lone pair of electrons to form a dative (coordinate) covalent bond with the ions of d-block elements. Examples of complexes: – [Co(NH₃)₆]³⁺ – [Ni(CN)₄]²⁻ COLOURED COMPOUNDS WHY????? d-orbitals are oriented in five planes. In an isolated atom, all d-orbitals are at the same energy Due to the effect of ligands on them, d-orbitals are divided into two groups, t-orbitals and e-orbitals. What happens???? Transition metals complexes can be octahedral (six ligands) or tetrahedral with four ligands. Octahedral shapes Due to the orientation of d-orbitals, two of them will point direct on the point of charges (e-orbitals), remaining of them between the charges (t-orbitals). T-orbitals are at a lower energy than e-orbitals. Tetrahedral shapes In these shapes two e-orbitals point between the charges while three torbitals point more directly on the charges t-orbitals are at a higher energy than e-orbitals. Effect When light passes through these compounds, electrons from a lower energy d-orbital absorb a photon of energy and are promoted to higher energy d-orbitals The energy absorbed is equivalent to the energy difference between the two sets of orbitals. Energy is given by ∆E= hv v is the frequency of light absorbed Since light of a certain frequency is absorbed, the light that comes out looks coloured because it lacks some colour. The colour of the compound is the complementary of the one that was absorbed. Factors affecting the colour Oxidation state of the central metal The nature of the ligand Exceptions Sc3+ and Ti4+ Since their d-orbitals have no electrons which could be promoted. Zn2+ and Cu+ Their d-orbitals are full. Transition metals and their Ions as catalysts Catalysts A substance which alters the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation energy. Different catalysts Homogenous catalysts This means the catalyst is in the same state of matter as the reactants. Heterogeneous catalysts This means the catalysts is in a different state of matter from that of the reactants Why and how transition metals work as both heterogeneous and homogeneous catalysts? HETEROGENEOUS By using their 3d and 4s electrons to form weak bonds with small reactant molecules, they provide a surface for the reactant molecules to come together with the right orientation. Electrons from the bonds making up the molecules are used to bond to the metal atoms. This is what weakens the bonds within the molecules thus lowering the activation energy needed for them to react HOMOGENEOUS Ions of transition metals show variable oxidation states which allow them to be effective homogeneous catalysts more especially in redox reactions Such characteristics are of fundamental biological importance in enzyme based reactions Demonstration of heterogeneous catalysis Nickel used in hydrogenation of ethene to ethane. The nickel metal absorb hydrogen and ethene onto its surface and align them so that they are in the right orientation to react. Nickel catalysis Other examples Iron (Fe) in the Haber process: N2 (g)+ 3H2 (g) ↔ 2NH3(g) MnO2 in the decomposition of hydrogen peroxide 2H2 O2 (aq) → 2H2O(l) + O2 (g) • V2O5 in the Contact process; 2S O2 (g) + O2 (g) ↔ 2SO3 (g) First example of homogeneous catalysis Fe2+ in heme: Oxygen is transported through the bloodstream by forming a weak bond with the heme group of hemoglobin This group contains a central Fe2+ ion surrounded by 4 nitrogen atoms The O2 – Fe2+ bond is easily broken when oxygen needs to be released Second example Co3+ in vitamin B12 Part of vitamin B12 consists of octahedral Co3+ complex 5 of the sites are occupied by nitrogen atoms leaving the sixth for biological activity Cobalt in vitamin B12 Sources Atkins P.W and Beran .J. A, General chemistry , Scientific American Books,( 1992). http://www.chemguide.co.uk/inorga nic/complexions/colour.html Course companion Chemistry Geoffrey Neuus pcc.ac.uk www.chm.bris.ac.uk