CH 421 Prelab 1 Spring 2011
advertisement

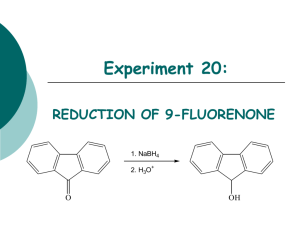
CH 421, Pre-lab 1 Spring 2011 Name__________________________________ Section ________________________________ Monitoring a Reaction with Thin-Layer Chromatography: Sodium Borohydride Reduction of Fluorenone (Pavia, Exp 5C, 40-41; refer to Pavia Technique 20 and McMurry Chapter 17) O OH NaBH4 MeOH 9-fluorenone 9-fluorenol 1) Which compound is more polar – fluorenone or fluorenol? 2) Will the product fluorenol have a higher or lower Rf on silica gel TLC than the starting material fluorenone? 3) The TLC eluent listed in the procedure is dichloromethane (also referred to as methylene chloride). If the spots have too high of an Rf (e.g. 0.8), would you add hexanes or methanol to the TLC eluent to lower their Rf values? 4) If the reduction reaction did not go to completion, 9-fluorenol and 9-fluorenone would both be isolated by precipitation from water. Considering their physical properties, list two different methods of purifying fluorenol from fluorenone: 1. 2. 5) If the progress of this reduction reaction was monitored by IR, the following would be observed: a. b. c. d. Disappearance of a peak at 3300 cm-1 and appearance of peaks at 1030 cm-1 and 1720 cm-1 Disappearance of a peak at 1720 cm-1 and appearance of a peaks at 1030 cm-1 and 3300 cm-1 Disappearance of a peak at 1030 cm-1 and appearance of peaks at 1720 cm-1 and 3300 cm-1 None of the above 6) Indicate “yes” or “no” whether fluorenol can be visualized on TLC using the following: UV (254 nm) ________ I2 ________ AgNO3 Ninhydrin KMnO4 ________ ________ ________ 2,4-dinitrophenylhydrazine ________ 7) The 1H NMR spectra of 9-fluorenone and 9-fluorenol are shown below. The aromatic peaks of both compounds fall within the 7-8 ppm region. If you examine a 1H NMR spectrum of 9-fluorenone, it should be obvious if there is any 9-fluorenol present. However, if you examine a 1H NMR spectrum of 9-fluorenol, how would you determine if there was any 9-fluorenone present, based only on the 1 H NMR spectrum? (Assume there is no NMR solvent peak overlap.) 9-fluorenone (CDCl3, 400 MHz) 9-fluorenol (CDCl3, 400 MHz) 8) Draw the products of the following benzaldehyde reductions, indicating specifically where deuterium is incorporated in the molecules. Complete the following table and include it, along with the other reaction information, in your lab notebook prior to the experiment. Where a line is drawn through a cell in the table, you do not need to include that data. Date of experiment Monitoring a Reaction with TLC: Sodium Borohydride Reduction of Fluorenone Reference: Pavia, D.L.; Lampman, G. M.; Kriz, G. S.; Engel, R. G. A Small Scale Approach to Organic Laboratory Techniques; Brooks/Cole Cengage Learning; Belmont, CA, 2011; pp 40-41. O OH NaBH4 MeOH fluorenone Name formula MW (g/mol) fluorenol mass fluorenone 0.400 g sodium borohydride 0.040 g _______ ______ mmol equiv d (g/mL) vol 1.00 ____ ____ _____ ____ ____ 8 mL ____ ____ Literature mp= _____ _____ mp (°C) bp (°C) ___ ____ methanol mol/L fluorenol product Theoretical yield = Theoretical mmol= _____ _____ ___ Note: One NaBH4 provides 4 equivalents of hydride. Thus, 0.25 mmol NaBH4 reduce 1 mmol of ketone. Procedure: Briefly describe procedure so that another person could reproduce this experiment. Do not include excess information – assume the reader is an organic chemist and understands general reaction set-up, work-up, isolation and purification procedures. Sample procedure: To a solution of fluorenone (0.400 g, ______ mmol) in MeOH (______ mL) was added NaBH4 (0.040 g, ______ mmol). The reaction mixture was stirred at room temperature for _______ minutes, after which silica gel TLC indicated it was complete (provide TLC eluent, method of visualization, and Rf of starting material and product). Water (______ mL) was slowly added to the reaction mixture, then the mixture was heated to reflux for _______ minutes. The reaction mixture was allowed to cool to room temperature then was placed on ice. The resulting solid was isolated by vacuum filtration and washed with cold MeOH/H2O (4:1, 3 x 2 mL). After drying the product was obtained as a _______________ (______g, ______% yield). Melting point _____ °C. 1H NMR and IR data were consistent with the desired product. Data: Sketch the TLC plate – indicate solid phase (silica gel), the eluent used (CH2Cl2), method of visualization, and Rf values. (Do not attach TLC plate to notebook.) List melting point range compared to literature value Tape or staple the interpreted IR and 1H NMR spectra into notebook Conclusions: Comment on the effectiveness of TLC for monitoring this reaction and the yield of product obtained. Assign the peaks in the IR and 1H NMR spectra. Comment on the yield and the purity of product based on melting point and 1H NMR.