Experiment: Using Hess Law (SL/HL): hydration of copper sulphate
advertisement

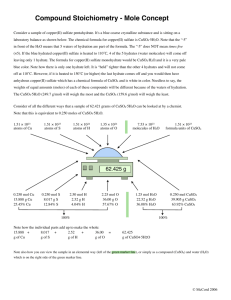
HessLawmagnesiumsulphatePage 1 of 3 IB CHEMISTRY INTERNAL ASSESSMENT Experiment: Using Hess Law (SL/HL): hydration of copper sulphate Aim To determine the enthalpy change for the reaction CuSO4 (s) + 5H2O (l) CuSO4.5H2O (s) Introduction It is impossible to measure the enthalpy change for this reaction directly because if we add 5 moles of water to 1 mole of copper (II) sulphate, we do not produce hydrated copper (II) sulphate crystals. These can only be made by crystalisation. However, you can measure the enthalpy change of solution for the following two solids shown in the equation below: CuSO4 (s) + 100H2O (l) CuSO4 (aq, 100H2O) CuSO4.5H2O (s) + 95H2O (l) CuSO4 (aq, 100H2O) Assessment opportunities: practice data collection and processing Aspect 1 Aspect 2 Defining the problem and selecting variables Aspect 3 Controlling variables Levels/marks Developing a method for collection of data Formulates a focused Designs a method for the Develops a method that allows problem/research question and effective control of the variables. for the collection of sufficient identifies the relevant variables. relevant data. Formulates a problem/research question that is incomplete or identifies only some relevant variables. Designs a method that makes some attempt to control the variables. Does not identify a problem/research question and does not identify any relevant variables. Designs a method that does not Develops a method that does control the variables. not allow for any relevant data to be collected. 0 1 2 0 1 Develops a method that allows for the collection of insufficient relevant data. 2 0 1 2 Requirements and materials safety goggles distilled water spatula teat pipette CuSO4 (s) thermometer, 0 – 50 C balance CuSO4.5H2O (s) 2 polystyrene cups with lid Procedure A: Heat of solution of CuSO4 (s) (=dried/anhydrous) Complete/2 Partial/1 Not at all/0 total: /6 HessLawmagnesiumsulphatePage 2 of 3 Use 0.10 mol of anhydrous copper (II) sulphate and 100 cm3 of water. Procedure B: Heat of solution of CuSO4.5H2O (s) Use 0.10 mol of hydrated copper (II) sulphate but using only 91 cm3 of water as the hydrated salt already contains 9 cm3 (0.5 mol) of water. Data collection and processing Under the heading “Measurements and Observations”, design an appropriate results table which contains all relevant raw data which will allow you to calculate the enthalpy change using Hess’s Law. Under the heading “Calculation” , use the raw data recorded in the above results table to calculate the enthalpy change for the reaction given. Draw an enthalpy cycle diagram. Show clearly how all raw data has been processed and carry out a propagation of uncertainty. Assessment opportunities: conclusion and evaluation Aspect 1 Aspect 2 Concluding Aspect 3 Evaluating procedure(s) Levels/marks Improving the investigation States a conclusion, with Evaluates weaknesses and justification, based on a limitations. reasonable interpretation of the data. Suggests realistic improvements in respect of identified weaknesses and limitations. States a conclusion based on a Identifies some weaknesses reasonable interpretation of the and limitations, but the data. evaluation is weak or missing. Suggests only superficial improvements. States no conclusion or the conclusion is based on an unreasonable interpretation of the data. 0 1 2 1 2 0 1 Partial/1 Not at all/0 Identifies irrelevant weaknesses Suggests unrealistic and limitations. improvements. 0 Complete/2 2 total: /6 The accepted value = - 62 kJ mol-1. Conclusion and Evaluation (CE) !st aspect: Write a conclusion (= reply to your aim) based on the experimental results. Indicate how you have used/interpreted the experimental results to arrive at your conclusion. Give an explanation for your conclusion using your Chemistry knowledge. Compare your experimental results with literature values or expected values (if available) and state if possible a % error and if it is positive (more) or negative (less) 2nd aspect: HessLawmagnesiumsulphatePage 3 of 3 Evaluation of result Describe any limitations in the way you have interpreted your results e.g. have you ignored any variables which you have not measured or could not have measured? Evaluation of procedure (=materials/equipment + design/method) consists of: Identification of systematic errors which are errors due to the quality of the equipment and materials, poor experimental design and ‘incorrect’ use of the equipment. These errors cannot be calculated and are also difficult to evaluate. However, these systematic errors can be reduced by using better equipment/materials or improved experimental technique. a. Evaluation of materials/equipment: Measuring tools improperly calibrated? Accurate enough? Incorrect concentration of reagents? Impure reagents? Amounts of reagents used large enough? b. Evaluation of method: Are there any weaknesses in the method which could have caused an error greater than the % uncertainty? Did you make any errors when carrying out the experiment e.g. did you not do some thing which you should have done or did you do it incorrectly? Were some variables not controlled? Were readings duplicated? c. Evaluation of result: describe any limitations to the way you have interpreted your results e.g. have you ignored any variables which you could have measured or have not measured? Have you used all the raw data? For each identified limitation in (a) or (b), weakness or error indicate the direction of its effect on the experimental result i.e. would it have caused your experimental result be more or less. Limitation/weakness How much did it affect my result Materials/equipment Design/method Evaluation of quality of the result: 3rd aspect For each suggested weakness, limitation or error suggest improvements Both the 2nd and 3rd aspect can be done using a table as shown below. There is no need to always fill something in each row. Limitation weakness 1. 2. improvement