Prepare 0.2M CuSO4 Solution: Step-by-Step Guide
advertisement

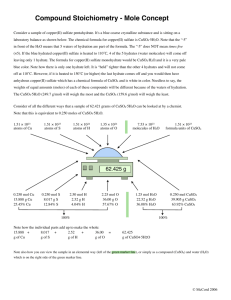
HOW TO PREPARE 0.2M CuSO4 SOLUTION Molar (M) solutions are based on the number of moles of chemicals in 1 liter of water. A mole consists of 6.02x 1023 molecules. The molecular weight (MW) is the weight of one mole (6.02x 10 23 molecules) of a chemical. The molecular weight of CuSO4 (anhydrous) is 159.62g/mol. The molecular weight of CuSO4. 5H20 (pentahydrate) is 249.68g/mol. You can use either form of Copper Sulfate to make the solution. The hydrate form does not dissolve as quickly so if you are using this you should prepare your solution in advance. You can use warmer water and a stir plate to help it dissolve quicker. The following calculations are for pentahydrate copper sulfate CuSO4.5H2O. If you use anhydrous CuSO4 make sure you use the proper molecular weight for your calculation. Calculations If we want 100ml of a 0.2M solution of CuSO4, we need to calculate how many grams of CuSO4.5H2O are needed. Given: require 100 mL soln require 0.2 M CuSO4 MW (molecular wgt) of CuSO4.5H2O = 249.68 g/mol We need to find: g of CuSO4.5H2O Use: mol CuSO4.5H2O = Molarity x volume Molarity = mol/1L Vol = 100.0 mL g CuSO4.5H2O = 100.0 mL soln x 1L /1000mL x 0.2mol/ 1L soln x 249.68 g CuSO4.5H2O /1mol = 4.99 g CuSO4.5H2O So you would dissolve approximately 5g of the Copper Sulfate (hydrate form) crystals in 100ml of water to make a 0.2M solution. 100ml of solution allows for about 15ml in each of your 6 test tubes. If you find that the crystals of copper sulfate are too big, don’t panic, you do not need to grind them or break them. You can make a larger amount of the 0.2M solution and use it later. Weigh the chunk then use a ratio to deterimine the amount of water to make the equivalent ratio. Use what you need and store the rest of the 0.2Msolution (clearly labeled in a tight lid container) for later use. For example, the chunk weighs 10.4g. The ratio is: 5g/100mL = 10.4g/ unknown mL rearranging terms: unknown ml = 10.4 g x (100mL/5g) = 208mL Therefore if you dissolve your 10.4g chunk in enough water to make 208 mL of solution you again have a 0.2M solution. Use the 100mL for the demo now and store the rest safely (clearly labeled in a jar with a tight fitting lid) for later use.