Items-to-review-for-Moorpark-College-exam
advertisement

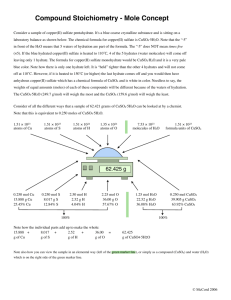
Items to review for Moorpark College exam 1. Review cheese lab concepts (understand basic scientific principles such as variables, control group, experimental group, hypothesis, etc.) 2. Chapters to Review a. Chapter 1—safety (look up OSHA, ASTM, EPA, NRC), MSDS, FDA pathway, manufacturing pathway, scientific notation, b. Chapter 2—amino acids, transcription/translation, DNA, mRNA, tRNA, proteins, media growth, components of media c. Chapter 3—concentration of solutions, conversion (% to decimal, quantity need to create a solution, serial dilutions, units, review quarter 1 test (attached) d. Chapter 5—protein structure (4 levels), e. Chapter 7—memorize table 7.1, read about pH, spectrometer (know all key voacb in 7.1) f. Chapter 8—transformation (gfp lab), Spinner Flasks, Roller Bottles, Bioreactors (page 228-229) g. Chapter 9—chromatography (know all the bold words and how each type of chromatography works) 3. pH review (read chatper 7 carefully) a. definition b. hydrogen ion concentration of solutions when given the pH c. what makes a pH higher d. table 7.1 4. Vocab you need to know not from a specific chapter: a. Restriction enzyme b. DNA polymerase c. DNA ligase d. Agar plates Biotechnology Quarter 1 Exam Conversions 1. Covert the following values (5 pts): a. 12.5 µL =__0.0125___mL b. 3.05 mL=__3050__µL c. 0.45 L=__450____mL d. 1.25 g = __1250____mg e. 989 µg = __0.989___mg 2. How much gelatin solution is needed to make 15mL of 12mg/mL gelatin solution? 180 mg=0.18 g 3. How much glucose is needed to make 50mL of 2.5% glucose solution? 1.25 g 4. How much CaCl2 is needed to make 125 mL of 0.55 M CaCl2 solution? 7.63 g 5. Report the following % mass/volume concentrations in g/mL units? (3pts.) a. 10% NaOH=___0.1__g/mL NaOH b. 5% CuSO4 =__0.05___g/mL CuSO4 c. 1.25% gelatin =_0.0125____g/mL gelatin 6. Suggest a method to make a 0.5 M solution from a 1 M solution. The .5 M solution could have been made b diluting the 1 M solution. Since .5 M is one –half the concentration of 1 M, use equal parts water and 1 M solution. For example, mix 2.5 mL of the 1M solution with 2. 5 mL of water. 7. Diagram how to prepare the solution below. Make sure the order of events and amounts necessary are clear. (12 pts) Solution Diagram 25 mL of 2.5 g/mL NaCL solution 62.5 g NaCl 2 L of 0.5 g/mL dextrose solution 1000g 200 mL of 8% NaCl solution 16g 0.75 L of 5% dextrose solution 37.5g 125 mL of 10 M NaOH 50 g 75 mL of 0.1 M NaCl 0.0438 g 8. Calculate the mass/volume concentration in each tube and the % mass/volume concentration in each tube and record these data in the table. (6 pts.) Molar Concentration (g/mL) Concentration (%) Concentration of each tube 5 mL of 1 M .25 25% CuSO4 5 mL of 0.5 M .125 12.5% CuSO4 5 mL of 0.1 M 0.025 2.5% CuSO4