Calculation of quantities required for a chemical reaction
advertisement

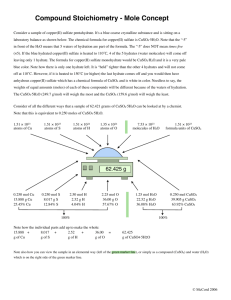
Calculation of quantities required for a chemical reaction, % yield and atom economy Suppose you want to make 50g of the blue hydrated copper(II) sulphate crystals o . Your reactants are dilute sulphuric acid and the black solid copper(II) oxide. o You can use copper(II) carbonate, but this is not a pure simple compound and the predictive nature of the calculations will not be as good. o copper(II) oxide + sulphuric acid ==> copper(II) sulphate + water o (i) CuO(s) + H2SO4(aq) ==> CuSO4(aq) + H2O(l) o on crystallisation you get the blue hydrated crystals of formula CuSO4.5H2O o so strictly speaking, after evaporation-crystallisation the equation is o (ii) CuO(s) + H2SO4(aq) + 4H2O(l) ==> CuSO4.5H2O(s) o How much copper(II) oxide is needed? o A 'non-moles' calculation first of all, involving a reacting mass calculation. o The crucial change overall is CuO ==> CuSO4.5H2O o (Note: In reacting mass calculations you can often ignore other reactant/product masses) o Atomic masses: Cu = 64, S = 32, H = 1, O = 16 o Formula masses are for: CuO = 64 + 16 = 80, CuSO4 = 64 + 32 + (4 x 16) = 160 o and CuSO4.5H2O = 64 + 32 + (4 x 16) + [7 x (1+1+16)] = 250 o The crucial reacting mass ratio is: 80 ==> 250 since formula ratio is 1:1 in the equation. o Therefore, theoretically, to make 50g of the crystals (1/5th of 250), o you need 1/5th of 80g of copper(II) oxide, o and 80/5 = 16g of copper(II) oxide is required. o However, in reality, things are not so simple because the method involves adding excess copper(II) oxide to the dilute sulphuric acid. (see salt preparation method (b)) o So in practice you would need to take more of the CuO to get anything like 50g of the salt crystals. o There is another way to calculate the quantities required based on the acid. o How much dilute sulphuric acid (of concentration 1 mol dm-3) is required? Mol = mass in g / formula mass, so moles of CuSO4.5H2O required = 50/250 = 0.2 mol (see basics of moles) Therefore 0.2 mol of H2SO4 is required (1/5th mol), since the mole ratio CuO : H2SO4 is 1 : 1 in the equation. 1dm3 (1000 cm3) of a 1 mol dm-3 solution of contains 1 mole (by definition see molarity page) Therefore 1/5th of 1dm3 is required, so 200 cm3 of 1 mol dm-3 dilute sulphuric acid is required, or 100 cm3 of 2M dilute sulphuric acid. (2M is old fashioned notation for 2 mol dm-3 as seen on many laboratory bottles!) You would then add copper(II) oxide in small amounts until no more dissolves in the warm-hot acid and the excess black powder is filtered off. There is no need to weigh out an exact amount of copper oxide. o Suppose after carrying out the preparation you finally crystallise 34g of pure the blue crystals of CuSO4.5H2O and weigh the product when dry. o What is the 'atom economy' of the preparation? (you need to refer to equations (i)/(ii) at the start of section 14.5 Atom economy = useful theoretical products x 100/mass of all reactants based on equation (i) Atom economy = mass CuSO4 x 100 / (mass CuO + H2SO4) = 160 x 100 / (80 + 98) = 16000/178 = 89.9% based on equation (ii) the atom economy is 100% if you include water as a 'reactant', can you see why? What is the % yield? i.e. comparing what you actually get with the maximum possible, i.e. a 'reality check'! % yield = mass of product obtained x 100 / theoretical mass from the equation % yield = 34 x 100 / 50 = 68%