chem lab write up hydrates
advertisement

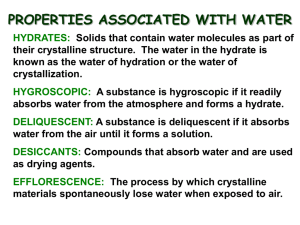
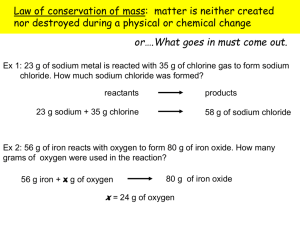
Elyssa Edgeton College Chemistry Wentzel Hour 3 11/11/14 Discussion of Theory Our lab groups heated the hydrate CuSO4 * xH2O in order to evaporate the water in the compound, and measured the mass change that occurred. This lab allowed us to observe how hydrates, crystalline structures containing water, can be turned into anhydrous compounds once the water is removed. We also applied our knowledge of how to find chemical formulas with mass percents in order to find the chemical makeup of our hydrate. We assumed that water was the only component evaporating from the compound, and caused all of the mass change. We also assumed that the anhydrous compound, CuSO4, was the only remaining component, and had not changed from its original form. Sources of Error Our group calculated the ratio of moles of H2O to CuSO4 and found that there was about a 5:1 ratio between the compounds. We did have a 1.76% error, however, meaning there was slightly more mass from water than the integer we expected. This is probably due to the fact that we did not have enough time to fully heat and dry the compound until there was no mass change, and nearly all of the water was gone. This would mean that our observed percent of water to CuSO4 was actually less than it should be. Our scale was also not accurate to the 4th decimal, which would affect our results’ accuracy as well.