Molarity Homework Answers: Chemistry Solutions
advertisement

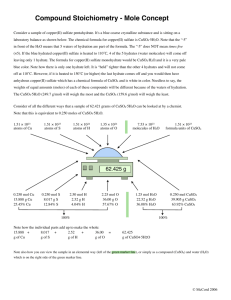
Answers for Homework on Molarity due for Friday, January 11th, 2013. p. 482 #10. How many moles of ammonium nitrate are in 335 mL of 0.425 M NH 4NO3? 0.425 mol NH4NO3 x __1 L __ x 335 mL L solution 1000 mL #12. = 0.140 mol NH4NO3 How many milliliters of a solution of 4.00 M KI are needed to prepare 250.0 mL of 0.760 MKI? Diluting the 4.00 M KI to M1 V1 0.760 M KI determining the amount of water needed. = M2 V2 (0.760 M) (250.0 mL) = (4.00 M) V2 solve for the unknown volume V2 47.50 mL = 47.50 mL of the 4.00 M of KI should be placed into the 250 mL volumetric flask. After that is in the flask water is added until it reaches the etched line (total volume of 250.0 mL). #19. Calculate the molarity of a solution containing 400 g CuSO4 in 4.00 L of solution. 400 g CuSO4 x 1 mol CuSO4 = 4.00 L 159.6 g CuSO4 #20. 0.630 mol CuSO4 L (solution total) = 0.630 M CuSO4 How many moles of solute are present in 50.0 mL of 0.20 M KNO 3? 0.20 M KNO3 remember that M = mol solute (KNO3) L solution 0.20 mol solute (KNO3) L solution x 1L x 1000mL 50.0 mL = 0.0100 mol KNO3