Separating Mixtures: Chemistry Class Notes
advertisement

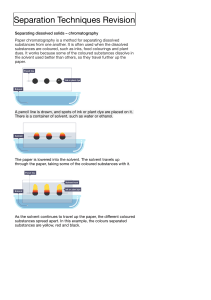
Academic Chemistry Mr. Gensits Class Notes 3/11/2010 Separating Mixtures Mixtures can be separated into their constituent substances through physical means. There are many physical processes that can be used to separate mixtures. The easiest and most effective technique depends upon the physical characteristics of the substances that make up the mixture. The following techniques can be considered when attempting to separate a mixture: 1. Filtration - method used to separate a solid from a fluid. The solid is separated when the mixture is placed on a medium that retains the solid while allowing the fluid to pass through. 2. Decantation – technique that can be used to separate an insoluble solid from a liquid. The liquid is carefully poured away leaving the sediment behind. 3. Chromatography – a mixture is dissolved in a “mobile phase” which passes over or through a “stationary phase.” The separation occurs due to the relative affinities for the dissolved substances to the two phases. Paper chromatography is often used to separate colored dyes in a solution. 4. Fractional Crystallization - is a method of separating dissolved solids due to their differences in solubility in the solvent. If two or more substances are dissolved in a liquid, they will crystallize out of solution at different rates at different temperatures depending on their relative solubility. 5. Magnetism – a magnetic substance can be physically pulled out of a mixture with a magnet. 6. Distillation – a dissolved solid and a liquid solvent can be separated and isolated from each other by boiling away the solvent and condensing the resultant vapor in a separate container. 7. Fractional Distillation – two liquids can be separated from one another by using their boiling points. If the difference in the boiling points of the two liquids is greater than 25oC then a simple distillation may be employed. 8. Centrifugation – process that utilizes centrifugal force to separate substances with different densities.