Separation of Mixtures KEY
advertisement

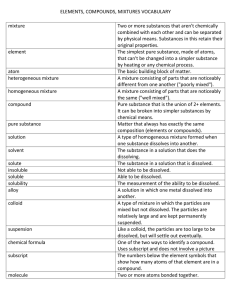
Name ______________________________________________ Kaspriskie Notes 1.2 Separation Techniques Vocabulary – Use your textbook or the Internet to define the following terms Word Filtration Definition A process that separates a solid from the liquid in a heterogeneous mixture Crystallization A process that separates a dissolved substance from the liquid in a homogeneous mixture based on a difference in boiling points. Distillation A process used to separate components of a mixture (usually homogeneous) based on a difference in boiling points. Video 1.2a: Filtration Sketch a diagram of the laboratory set up 1. What do you use this technique to separate? A solid from a liquid 2. Can you collect all substances from the original mixture? Yes Video 1.2b: Crystallization Sketch a diagram of the laboratory set up 1. What do you use this technique to separate? Dissolved substances from the liquid they’re in 2. Can you collect all substances from the original mixture? No, only the dissolved particles (as crystals). Liquid evaporates into the atmosphere. Video 1.2c: Distillation Sketch a diagram of the laboratory set up 1. What do you use this technique to separate? Two liquids OR a dissolved substance from a liquid 2. Can you collect all substances from the original mixture? Yes.