Objective
advertisement

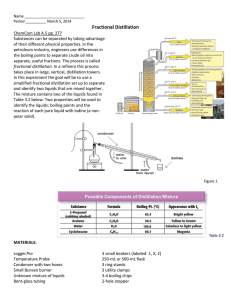
Lab 4 Fractional Distillation Objective To separate a mixture of two liquids by collecting vapors at their boiling point. Experiment Pour about 50 mL of mixture into a grad. cyl. Soak a paper strip in the mixture. Hold the paper over the burner to test for flammability. Record results Time (min) 0 0.5 1.0 1.5 … Heat mixture gradually and collect 2 fractions Temp (°C) Odor Mixture Fraction 1 Fraction 2 Fraction 3 (leftover in flask) Flammability Analysis 1. Construct a graph of temperature vs. time for the heating of the solution. Place the time along the horizontal and the temperature along the vertical axis. Only include the temperature range you recorded along the vertical for a more accurate graph. Label the graph. Results - Questions 1. Why is this method of separation called “fractional” distillation? 2. What would the flat line in your graph indicate? 3. Propanol is an compound that has a boiling point around 98̊C. Explain why a mixture of propanol and water would be difficult to separate by fractional distillation.