PowerPoint Presentation - Physical & Chemical Properties & Change
advertisement

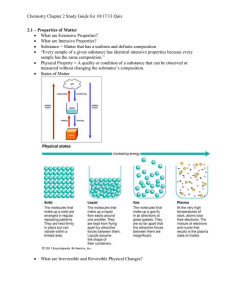
Matter: Properties & Changes Chapter 2 notes (2.1) Physical Properties of Matter can be observed or measured without changing the composition of the sample Examples: color, phase, melting or boiling point, density, hardness, odor, taste • • • • States of Matter Solid (s) - definite volume and shape Liquid (l) - definite volume, but not shape Gas (g) - no definite volume or shape Plasma - highly ionized gas (s) (l) (g) sToP & tHinK • Which state or states of matter take(s) the shape of its container? • Which state or states of matter can change in volume (without adding or subtracting from the sample)? Physical Changes • Physical changes alter a substance without changing its composition sToP & tHinK 1. Which of the following are physical properties? • • • • liquid at room temperature reacts with metals to form H2 gas acids and bases combine to form water and a ‘salt’ boils at 145 degrees Celsius (2.2) Mixtures… • Mixture - a combination of two or more pure substances, in which each substance retains its individual chemical properties • Heterogeneous mixture - does not blend smoothly, individual substances are distinct • Homogeneous mixture - solution; has a constant composition throughout sToP & tHinK • Heterogenous or Homogeneous? (1) (2) Separating Mixtures • Filtration - porous barrier separates solid from liquid • Distillation - liquids separated by differences in boiling point • Crystallization - forms pure solids from dissolved substances • Chromatography - separation based on ability to travel or be drawn across a material filtration distillation crystallization chromatography sToP & tHinK What separation technique is shown in the following pictures? A B (2.3) Elements & Compounds • Elements - pure substances that cannot be broken down into simpler substances – on the Periodic Table! • Compounds - two or more elements bonded together that can be broken down sToP & tHinK 1. In your own words, what is the difference between an element and a compound? 2. Classify the following as being elements or compounds a) b) c) d) e) Sodium Sodium chloride Oxygen Carbon dioxide Copper (2.4) Chemical Properties • the ability of a substance to undergo chemical change • Examples: “reacts with oxygen to form rust”, “forms a deep blue solution when in contact with ammonia” Chemical Changes • Chemical changes alter the composition so that a new substance forms • Evidence of chemical change: formation of a gas or solid (precipitate), smoke, fire, an odor, temperature change, color change – Law of Conservation of Mass: composition changes, but mass doesn’t sToP & tHinK • Substance A is a yellow liquid and substance B is a blue liquid. The two are mixed and form a green liquid and a white solid. – What evidence suggests a chemical change occurred? – What other things could you look for to determine if there was a chemical change? sToP & tHinK - which shows physical change and which shows chemical change? • link to change animation #1 • link to change animation #2