How to do long questions
advertisement
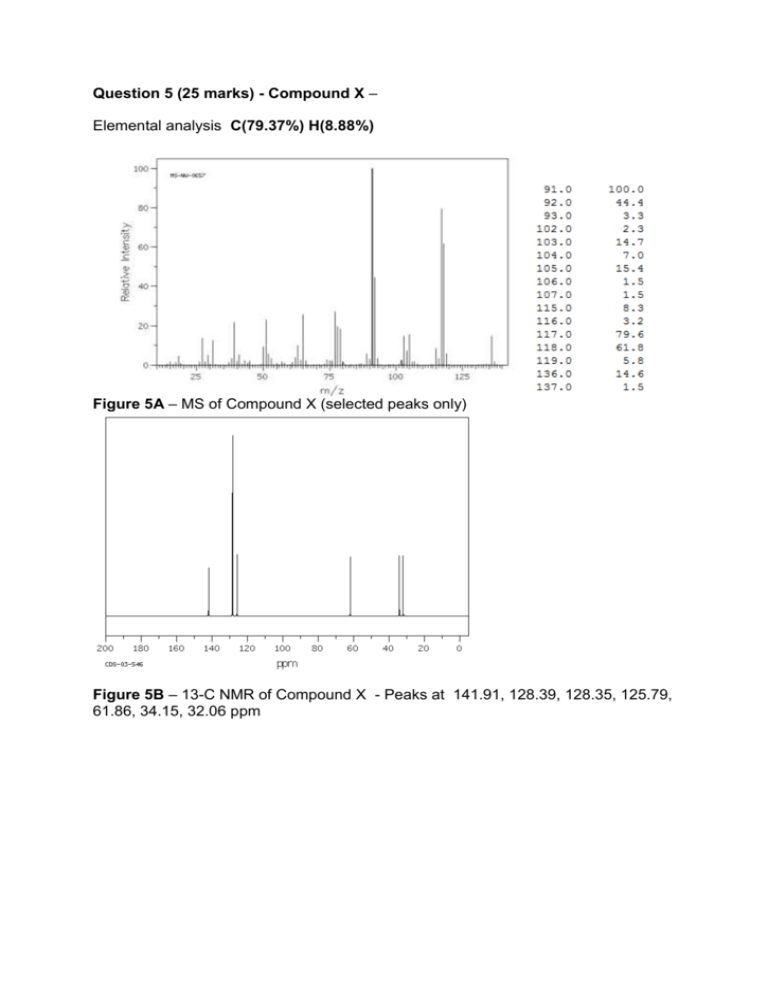
Question 5 (25 marks) - Compound X – Elemental analysis C(79.37%) H(8.88%) Figure 5A – MS of Compound X (selected peaks only) Figure 5B – 13-C NMR of Compound X - Peaks at 141.91, 128.39, 128.35, 125.79, 61.86, 34.15, 32.06 ppm Figure 5C – 1H- NMR of Compound X Peaks: 7.04-7.39 ppm (5H, complex); 3.625 ppm (2H, triplet, J=6.2 Hz); 2.674 ppm (2H, triplet, J= 8 Hz); 2.02 ppm (1H, singlet); 1.87 ppm (2H, multiplet) Figure 5D – IR spectrum of Compound X 1. Start with the elemental analysis and Rings and Double bonds – at least get a few marks for doing this mechanical step 2. Look at the general appearance of the spectra e.g. aromatic vs aliphatic – can tell quite easily from MS (fewer vs more fragmentation), 13C NMR (120-140 ppm), 1H-NMR (7-8 ppm) e.g. presence of carbonyl group – can tell easily from 13C-NMR (200 ppm), IR (1700 cm-1) with NMR it is possible to give a real negative conclusion e.g. if you don’t see peak at 200 ppm, it is quite valid to say no carbonyl group with MS and IR it is not so easy e.g. if you don’t see a broad peak at 3300 cm-1 it may not necessarily mean there is no OH group. By the end of this step, you should have a rough idea of the types of functional groups you have. This should be half the marks for the question already. 3. At this point, there are a number of approaches – this is not the only one. Look at the coupling patterns. I think coupling patterns are a more ‘fool-proof’ assignment rather than looking at the chemical shift because chemical shift regions overlap By the end of this you should have solved the structure – about ¾ of the question done. 4. Assign as much of the spectra as you can This is where your textbook or reference material which you bring in is helpful 1H NMR – try to assign all peaks using the chemical shift and check that the number of H is correct. 13C – NMR – try to assign all peaks Sometimes it is not possible to assign all peaks e.g. the aromatic ring – so don’t try to do everything. IR – try to assign those peaks which are consistent with the main functional groups – again it is not expected to assign all peaks. MS - try to assign those peaks which are consistent with the main functional groups e.g. if aromatic compound – tropylium cation, alcohols – dehydration product, alpha-cleavage products, ketones – alpha cleavage, MacLafferty arrangement products End