Solutions NMR Spectroscopy
advertisement

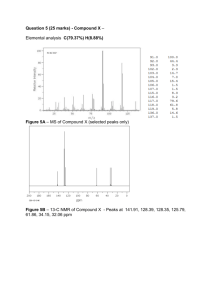
Additional Problems for practice. 1. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:M+ = 116 IR: weak absorption at 2190 cm-1 medium absorption at 1600cm-1 1H NMR 5 2 7 13C 6 5 1 4 PPM 3 2 80 PPM 60 40 1 0 20 0 NMR 140 120 100 There are five aromatic protons at 7.1 ppm. This indicates a mono-substituted benzene ring, the IR absorption at 1600cm-1 (Ar-H stretch) confirms this. There is a singlet at 3.3 ppm accounting for 2 protons, thus this is a CH2 group with no protons on neighboring carbon atoms. There is a singlet at 1.8 ppm accounting for one proton, so this proton is attached to a carbon with no protons on neighboring carbon. The weak 2190 cm-1 absorption in the IR is indicative of a triple bond. 13C shows aromatic carbons between 120 and 140 ppm; the two peaks at 701 and 81 ppm must account for alkyne carbons; the peak at 25 ppm is an aliphatic carbon atom, but because it is desielded relative to a normal alkane, it must be next to an aromatic ring. Putting these pieces together, you may come up with the following structure: H H H mass=116 g/mol 1H NMR chemical shifts: 3.29 7.06 H H 7.14 7.07 H 7.06 1.82 7.14 13C 128.7 NMR chemical shifts: 129.1 H 138.9 H 80.4 26.1 125.8 70.9 H 129.1 128.7 b. Mass spectrum: M+= 82 molecular formula: C5H6 O IR: 1690 cm-1 (strong) with a shoulder at 1626 cm -1 1H NMR 6 13 C 5 4 NMR 220 200 180 160 140 PPM 3 120 100 PPM 2 80 1 60 40 0 20 0 The 13C absorption at 208 ppm can only indicate the present of a ketone carbonyl carbon; the peaks at 135 ppm and 165 ppm are likely to be alkene carbons; Now the IR indicates a strong 1690 cm-1 which is definitely C=O, but it is conjugated since the ordinary C=O stretch occurs at 1710 cm-1. The shoulder at 1626 cm-1 indicates the alkene that is conjugated with the carbonyl. Indeed, the 1H NMR confirms this assignment: the absorptions at 6.1 and 6.6 ppm are indicative of two alkene protons. One of the alkene protons shows up as a doublet, indicating that there is only a single proton next door; the other alkene proton shows up as a doublet of triplets, which means that the proton is being split by two different types of protons: a single proton splits the absorption into a doublet, and a pair of other neighboring protons splits the resulting doublet into a pair of triplets. At 3.0 ppm we see a triplet, which indicates two protons neighboring this set of protons; its chemical shift indicates that these protons are next to the carbonyl unit, and the carbon atom to which they are attached absorbs at 40 ppm in the 13C NMR. The 3.0 ppm absorption also leans toward the quartet at 2.1 ppm, indicating the protons giving rise to these two absorptions are spin-spin coupled. The quartet at 2.1 ppm indicates protons that have three neighboring protons. The carbon atom to which these protons are bound absorbs at 25 ppm, deshielded relative to the alkane carbons, indicating that either an alkene or an aromatic ring (discounted based on molecular formula) is nearby. The molecular formula, C5H6O, indicates three degrees of unsaturation. We have already accounted for two: a C=O unit conjugated to a C=C unit. The remaining unsaturation can only be accounted for by a ring: O mass= 82 g/mol 1H NMR chemical shifts: O 6.07 H 2.98 H 6.57 13C 2.07 NMR chemical shifts: O 133.2 207.7 39.1 164.9 25.0 c. mass spectrum: M+ =136 IR: 1691 cm-1 (strong); 1600cm-1 ( medium) 1H NMR 10 13C 8 6 PPM 4 2 0 NMR 200 180 160 140 120 100 PPM 80 60 40 20 The IR again indicates the presence of a conjugated carbonyl: strong absorption at 1691 cm-1; there is no alkene shoulder, but the absorption at 1600 cm-1 is indicative of an aromatic ring, so we can assume that the carbonyl is directly bonded to (conjugated with) the aromatic ring. Looking at the 1HNMR, we can immediately tell what kind of carbonyl compound it is: the singlet at 9.8 ppm is characteristic of aldehyde protons. The carbonyl carbon of the aldehyde shows up at 191 ppm in the 13 C NMR. We can also tell the substitution pattern of the aromatic ring: there are only two sets of absorptions in the 7-8 ppm region, and both are doublets, which means 0 the protons giving rise to those absorptions have only a single other proton nearby. A high degree of symmetry is implied, thus we may guess that the aromatic ring is 1,4 disubsituted, with one of the substituents being an aldehyde. Now in the 13C spectrum, we see a strongly deshielded aromatic carbon shows at 168 ppm; this indicates that an electronegative element like oxygen, chlorine, bromine, etc is directly bound to that carbon atom. We also see the regular aromatic carbons at 128 and 131 ppm, respectively, but we can also see an unusually shielded absorption at 115 ppm, looking like roughly 2 carbons. Only an electron donating group on the aromatic ring could give rise to this type of shielding, so the other substituent on the aromatic ring is also an electron-donor. Looking back at the proton NMR, we see a large singlet at 3.8 ppm, probably indicating a methyl group attached to an oxygen (as we have seen in methanol), so a good candidate for the electron-donating group is the methoxy group. The methyl carbon directly bonded to oxygen absorbs at 57 ppm in the 13C spectrum. Putting all this information together gives us the following structure: Note: desielding effect of OMe due to oxygen's electronegativity: H H O O H3CO H3CO mass=136 g/mol 1H deshielded: 166.5 ppm NMR chemical shifts: 9.87 7.70 shielding effect of MeO (oxygen is a pi electron donor): H 6.96 H O O 7.70 O 6.96 H3CO 3.73 13C H NMR chemical shifts: 114.8 H 130.9 129.2 191.0 O O 166.5 shielded: 114.9 ppm H O H3CO 130.9 H 114.8 55.9 O H3CO H shielded: 114.9 ppm d. Mass spectrum: M+ = 176 molecular formula: C11H12O2 IR: 1735 cm-1 (strong); 1660 cm-1 (medium) 1600 cm-1 (medium) 1HNMR 7 6 5 4 PPM 120 100 PPM 3 2 1 0 13 CNMR 180 160 140 80 60 40 20 This compound shows aromatic protons (7.2-7.3 ppm) and alkene protons (6.2 and 6.6 ppm); we can confirm the presence of these functional groups in IR: the 1600cm-1 absorption and 1660 cm-1 absorptions indicate the aromatic C-H stretch and the C=C stretch, respectively. Now we also see the presence of an ester in the IR: a C=O 0 absorbing at 1735 cm-1 is characteristic of esters. The 13C NMR confirms the ester carbonyl carbon at 190 ppm; all the aromatic protons show up between 125 and 135 ppm. The absorption at 65 ppm indicates a group bound to an electronegative atom; since the molecular formula only has oxygen in it, this atom must be oxygen. Atom. Now the splitting pattern for the alkene protons tells us a lot of information:the deshielded alkene proton at 6.6 ppm is a doublet, indicating only one other proton next door; this other proton could be another alkene proton. Since there seems to be a large splitting (coupling constant ~ 15 ppm), we may be looking at a trans alkene. The other alkene proton at 6.2 ppm is a doublet of triplets, indicating two distinct types of protons next to this proton; one of the neighboring protons must be an alkene proton (trans) which the absorption to a doublet; two other protons are present which splits each of the doublet peaks into a triplet. Thus the alkene has a CH2 next to it. We can verify this if we look at the next absorption in the 1HNMR: at 4.7 ppm we see a doublet, which is the CH2 being split by a single neighboring proton (an alkene proton), and this chemical shift value indicates the presence of a nearby electronegative oxygen atom. The only other absorption in the proton NMR is a singlet at 2.0 ppm (probably a methyl group); its chemical shift (and also that of its corresponding carbon at at 20 ppm in the 13C NMR) indicate that it is next to a carbonyl. Putting all of this info together gives the following structure: O H CH3 O H mass=176 g/mol, C11H12O2 1H NMR chemical shifts: 6.62 7.30 H O 4.75 7.21 O CH3 2.01 H 7.14 7.30 6.25 7.21 13C 128.7 NMR chemical shifts: O H 126.4 66.6 135.2 129.7 123.9 H 128.0 126.4 128.7 170.3 O 20.8 2. Show a splitting tree for the Ha, Hb, and Hc protons in the following compound, and match your predicted multiplet patterns with the spectrum shown below. Indicate rough values for coupling constants on your diagram. O Ha Ha Hb Hb Hc Hc Ha 10 Hz splitting tree: 10 Hz 6 6 6 6 6 6 6 predicted multiplet pattern: O Ha Ha Hb 7 6 Hc 5 4 PPM 3 2 1 0