Answer Key for Test 1
advertisement

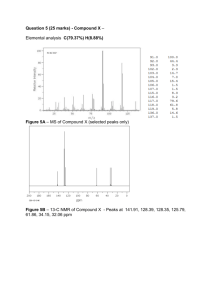
Exam 1 - CHM292 February 26, 2013 1. Draw the structures of the following compounds/functional groups (5 points each). (a) Pyridine (b) N-bromosuccinimide (c) Tosylate (d) Allyl bromide 2. Which reaction in each of the following pairs would you expect to be faster (5 points each)? (a) The SN2 displacement by I- on CH3Cl or on CH3OTos (OTos = tosylate) CH3OTos. Tosylate is a better leaving group than chloride. (b) The SN2 displacement by CH3CO2- on bromoethane or on bromocyclohexane Bromoethane. Bromocyclohexane is more sterically hindered and harder to displace. (c) The SN2 displacement on 2-bromopropane by CH3CH2O- or by CNCN- is a better nucleophile. (d) The SN2 displacement by HS- on bromomethane in toluene or in acetonitrile. Acetonitrile cetonitrile is a polar aprotic solvent. 3. Reaction of HBr with (R)-3-methylhexan methylhexan-3-ol leads to racemic 3-bromo-3-methylhexane. methylhexane. Draw the structure of 3-bromo-3-methylhexane, methylhexane, and draw a mechanism that accounts for the observed stereochemistry (10 points). H Br H3 C OH (R)-3-methylhexan-3-ol BrH 3C OH 2 H3 C planar carbocation intermediate + H3 C Br H3 C Br m 1:1 mixture 3-bromo-3-me ethylhexane 4. The structure of Viagra and its 1H NMR spectrum are shown below. (a) Match the peaks in the 1H NMR spectra with the protons in the compound, by labeling each peak AA M and using the same letter to label the protons on the structure. (15 points). l HH b C CH 3 d c N N O H HN N N H S H H i N H l HH O H H H H m CH3 H O CH3 e g H h f k a H 3C H m H O j (b) How many signals would you expect to see in the 13C NMR spectrum (5 points)? 20 5.. Answer all the questions and propose a structure of the u unknown nknown compound that is consistent with 1 13 the IR, H NMR, C NMR, and MS spectra shown (30 points). IR spectrum: (a) Mark 1 key peak in the IR spectrum and indicate what fun functional ctional group it corresponds to (5 points). Corresponds to a C=O double bond. Mass spectrum: M+ = 161 m/z (b) What is the molecular formula of the compound (Hint: the compound contains 1 nitrogen atom; only other atoms are C, H and O) (5 points)? C9H7NO2 1 H NMR spectrum: (c) Assign every one of the peaks in the 1H NMR to a proton in the molecule (5 points). H O H NC OCH3 H H red protons at 8.5 ppm blue protons at 7.6 ppm green protons at 3.8 ppm (d) Explain why the peaks between 7 and 8 ppm are split into doublets (5 points). They are from a para-substituted benzene ring. 13 C NMR spectrum: (e) Assign every one of the peaks in the 13C NMR spectrum to a carbon in the molecule. (Cluster of peaks around 77 ppm is from the solvent; peak at 0 ppm is from TMS; all other peaks need to be assigned) (5 points) (f) Draw the structure of the compound (5 points)