Answers to Chem 221 Spectroscopy Problems
advertisement

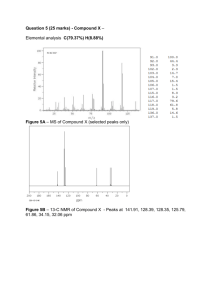
Answers to Chem 221 Spectroscopy Problems 1. MS: IR: m/z= 156, ∴ MW = 156. No M+2, ∴ no Br, Cl. No info about M+1, ∴no info about # of C’s. No peak around 1700 cm-1, ∴ no carbonyl No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) No broad peaks above 3000 cm-1, ∴no OH, NH N M R : Two types of hydrogens (two unique environments) δ (ppm) Area Multiplicity A 1.8 3 t B 3.2 2 q Based on IR this molecules appears at first glance to lack any functional groups, but 1H NMR δ 3.2 ppm implies some heteroatom. Combination of triplet & quartet is characteristic of -Et (CH2CH3), implies 2C’s & 5H’s. This has a mass of 29 and can be seen as a fragment in MS. 156 - 29 = 127. What has a weight of 127? Iodine. Correct Answer: CH3CH2I 2. MS: IR: m/z= 72, ∴ MW = 72. No M+2, ∴ no Br, Cl. No info about M+1, ∴no info about # of C’s. ν = 1720 cm-1, ∴ carbonyl (C=O) No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) No broad peaks above 3000 cm-1, ∴ no OH, NH N M R : Three types of hydrogen (three unique environments) δ (ppm) Area Multiplicity A 1 3 t B 2.2 3 s C 2.4 2 q We know from IR that molecule must contain (at least one) oxygen (OH), i.e. a mass of 16 MW - 16 = 56. What has a mass of 56? Guess: 4C’s & 8H’s (this is consistent with the total # of H’s in NMR). That gives MF: C4H 8O. How many degrees of unsaturation? Fully saturated would be Cn H 2n+2, or C4H 10, but we have C4H 8O, which means two H’s are missing, or 1 degree of unsaturation. That means 1 ring or one double bond is present. But we already know that it has a carbonyl so that must be the one degree of unsaturation. ∴ no other rings or double bonds. How many 4 C carbonyl compounds are there? 3: 1 ketone & 2 aldehydes O O H H O B C A The second one is ruled out because it has different types of H’s (4 different environments). The second and third are ruled out because it can not be an aldehyde: aldehyde carbonyls typically occur at larger wavenumbers (νC=O = 1740 cm-1, not 1720 cm-1), show two unusual CH stretches (ν = 2750 & 2850 cm-1) and show a very unique signal in the 1H NMR at δ 9 ppm. It must therefore be the first one. The NMR is consistent with the ketone. Protons near double bonds (C=C or C=O) appear ~2 ppm. Generic sp3 protons appear ~1 ppm. A has 2 neighbors and is a triplet, B has no neighbors and is a singlet, C has 3 neighbors and is a quartet. The areas are correct. Correct answer: O 3. MS: IR: m/z= 60, ∴ MW = 60. No M+2, ∴ no Br, Cl. No info about M+1, ∴no info about # of C’s. Broad peaks above 3000 cm-1, ∴ OH, alcohol likely No peak near 1720 cm-1, ∴ no carbonyl (C=O) No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) N M R : Four types of hydrogen (four unique environments) δ (ppm) Area Multiplicity A .9 3 t B 1.6 2 p or sx Multiplicity hard to tell C 3.1 1 s D 3.6 2 t We know from IR that molecule must contain (at least one) oxygen (OH), i.e. a mass of 16 MW - 16 = 44. What has a mass of 44? Guess: 3C’s & 8H’s (this is consistent with the total # of H’s in NMR). That gives MF: C3H 8O. How many degrees of unsaturation? Fully saturated would be Cn H 2n+2, or C3H 8, so it must be fully saturated. That means no rings or double bonds are present. How many 3 C alcohols are there? 2: 1-propanol & 2-propanol OH OH A B D C The second one is ruled out because it has only 3 different types of H’s (3 different environments). It must therefore be the first one. The NMR is consistent with the 1-propanol. Protons near heteroatoms (O, N, halogens) appear ~4 ppm. Generic sp3 protons appear ~1 ppm. Protons on O or N appear as broad singlets. A has 2 neighbors and is a triplet, B has 5 neighbors and is a sextet (although this is hard to see), C has neighbors but the coupling (splitting) is lost when H is attached to O or N, and D has 2 neighbors and is a triplet. The areas are correct - 3:2:2:1. 2-Propanol would have fewer peaks in NMR because by symmetry Me’s are equivalent. Also areas would be 6:1:1. Also splitting would give a doublet and a septet. Correct answer: OH 4. M F : IR: C6H 12O2 What could have 2 O’s? (a) diol (b) di-ether (c) diketone (d) di-aldehyde (e) carboxylic acid (f) ester No broad peaks above 3000 cm-1, ∴ no -OH. This rules out a & e. ν = 1735 cm-1, ∴ carbonyl (C=O) present. This rules out a & b. 1735 cm-1 is too high for most ketones. This rules out c. Only d & f are left (dialdehyde & ester). No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) N M R : Two types of hydrogen (two unique environments) δ (ppm) Area Multiplicity A 1.2 1 s B 3.7 3 s The NMR is frustratingly simple and at this point we can not even be certain of the functional group, but... How many degrees of unsaturation are there? Fully saturated would be Cn H 2n+2, or C6H 14, so it is missing 2H’s, which is one degree of unsaturation. This rules out di-aldehyde (d). It must be an ester. Moreover, there are no other double bonds or rings. How many 6 C esters are there? Too many! O O O O O O O O O O O O O O O O O O O O O O O O However only the two circled isomers give two singlets in NMR. t-Bu H’s are always singlets and Me’s are singlets when they are isolated (distant) from other H’s. All other isomers have too many peaks with lots of splitting. But which one these two is it? H’s attached to C’s attached to heteroatoms (such as O) are shifted substantially downfield to δ ~4 ppm. Therefore it must be the second one (methyl pivalate). Note: The NMR integration implies only 4 H’s. Actually the integrals are only the relative areas. Since we have the MF, we know the total area (# of H’s) should be 12, therefore each integral must be multiplied by 3. This give the correct areas - 3:9. O Correct Answer: 5. MS: IR: O m/z= 102, ∴ MW = 102. No M+2, ∴ no Br, Cl. No info about M+1, ∴no info about # of C’s. No broad peaks above 3000 cm-1, ∴ no -OH, -NH. ν = 1740 cm-1, ∴ C=O present. In the range of aldehyde or ester; too high for most ketones No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) N M R : Three types of hydrogen (three unique environments) δ (ppm) Area Multiplicity A 1.2 3 q (but see below!!) B 2.4 1 q C 4.2 1 q IR implies ester or aldehyde, but NMR is missing very distinctive aldehyde proton δ 9-10 ppm (aldehydes are the only peaks found in region 9-10 ppm), therefore must be ester. Ester group, -CO2- has a mass of 44. 102 - 44 = 58. What could have mass of 58? Guess: 4 C’s + 10 H’s. Possible MF: C5H 10O2. How many degrees of unsaturation are there? Fully saturated would be Cn H 2n+2, or C5H 10, so it is missing 2H’s, which is one degree of unsaturation. But the C=O is that degree of unsaturation, ∴ no other double bonds or rings. How many 5 C esters are there? 5: O O O O O #1 #2 O O O #3 O #4 O #5 NMR integrals shows a total of 5 H’s, but MF implies 10 H’s. ∴ Actual areas must be 2:2:6. Which of the above choices have 6 identical protons? #2 & #5. But this raises a problem. Those Me H’s must be doublets (not quartets), since each Me has one neighbor! We are in fact forced to rule out #2 & #5. #1, #2, #4, and #5 all have isolated Me’s, that is, Me’s that should appear as singlets, because they have no close neighboring H’s. This rules out: #1, #2, #4, and #5. By process of elimination it must be #3! How can we justify the Me’s of #3 as being a quartet? After all, our simple rules tell us it should be a triplet (they have 2 neighbors). In fact those two Me’s are not equivalent, one is closer to C=O, the other closer to O. In principle we should see 2 triplets. Why don’t we? Because the triplets occur at approximately the same chemical shift (d), they overlap slightly with each other. Two upfield peaks of one triplet exactly overlap with two downfield components of the other triplet, giving rise to an apparent quartet. The fortitous (or unfortuitous) overlap of peaks make this problem tricky. However, such overlap of peaks is not uncommon. The correct answer is ethyl propionate: 6. MS: IR: O O A C D B m/z= 136, ∴ MW = 136. No M+2, ∴ no Br, Cl. No info about M+1, ∴no info about # of C’s. Exceedingly broad peak ∴ -OH, but not -OH of alcohol, that would occur at ν > 3000 cm-1. This OH is so broad that it moves into the CH stretching region (~ 3000 cm-1) and thereby obscuring the CH stretches. This is characteristic of carboxylic acid (-CO2H ) . ν = 1705 cm-1, ∴ C=O present. Could be ketone or carboxylic acid, but in combination with observations above carboxylic acid is more likely. Possibly sharp peaks above 3000 cm-1, ∴ sp2 CH bonds (& ∴ C=C) possible, but hard to tell N M R : Three types of hydrogen (three unique environments) δ (ppm) Area Multiplicity A 3.6 5 s B 7.3 2 s C 10.6 1 s, broad For RCO2H, what is R? CO2H has a mass of 45. 136 - 45 = 91. Guess 7 C’s + 7 H’s. Possible MF: C8H 8O2 How many degrees of unsaturation are there? Fully saturated would be Cn H 2n+2, or C8H 18, so it is missing 10H’s (!), which is 5 degree of unsaturation. That’s a lot for such a small compound. Benzene ring is a good guess, because it has 4 degrees unsaturation (3 C=C + 1 ring). CO2H adds one degree unsaturation, bringing the total up to 5. Broad peak in NMR at δ 10.6 is characteristic of -CO2H (-CO2H is the only peak found in range δ 10-12 ppm). Confirms IR interpretation. What is peak so far downfield δ 7.3 ppm? Benzene ring. Protons on aromatic rings are typically the only peaks found in the region δ 7-8 ppm. Confirms guesses above. How many H’s on this particular aromatic ring? 5. Why 5 and not 6? Because it is monosubstituted: X What is X? Could it be -CO2H? No. This has the wrong MF, C7H 6O2. It is short by -CH2-. NMR also shows peak of area = 2. Because of the rules of valency, there is only one place to put -CH2-, between the aromatic ring & the -CO2H : B A C CO2H Why is the -CH2, so far downfield? Usually -CH2- adjacent to C=C or C=O is ~ 2ppm. But this -CH2- has 2 neighboring double bonds, one on either side & the effects are more-or-less additive, i.e. ~ 4 ppm. Why is there no coupling between H’s of aromatic ring? They all have nearly identical chemical shifts (δ’s) and identical H’s don’t split each other. 7. M F : IR: C9H 11N O2 (Would its molecular mass be even or odd?) What has 2 O’s? (a) diol (b) di-ether (c) diketone (d) di-aldehyde (e) carboxylic acid (f) ester (g) nitro How many degrees of unsaturation are there? C9H 11N O2 = “C9H 10” Fully saturated would be Cn H 2n+2, or C9H 20, so it is missing 10H’s (!), which is 5 degree of unsaturation. That’s a lot for such a small compound. Benzene ring is a good guess, because it has 4 degrees unsaturation (3 C=C + 1 ring). What is last degree of unsaturation? The IR spectrum was taken in CHCl3, which gives rise to unwanted peaks @ 3030, 1220 & 750 cm-1. Whats left? Two weak, broad peaks @ ν = 3400 & 3500 cm-1, typical of NH2. Peak @ ~1700 cm-1, ∴ C=O. This rules out (a) diol & (b) di-ether. N M R : Five types of hydrogen (five unique environments) δ (ppm) Area Multiplicity A 1.4 3 t B 4.1 B+C = 4 broad s overlaps with quartet C 4.2 B+C = 4 q overlaps with singlet D 6.6 2 d E 7.8 2 d IR C=O rules out diol & di-ether. Also no intense peaks @ 1500 & 1600 cm-1; rules out nitro. Exceeding broad -CO2H peak absent. Rules out carboxylic acid. Can not be dialdehyde because NMR shows no peak δ 9-10 ppm. NMR clearly shows aromatic peaks (benzene ring); that uses up 4 degrees unsaturation. Therefore can not be diketone (or dialdehyde) because they would bring total degrees of unsaturation to 6. Only the ester has one degree of unsaturation. But ester C=O typically occurs at 1740 cm-1. Why is this one at 1700 cm-1? It must be conjugated with C=C. In fact this is indirect evidence for C=C. (Unfortunately, any sp2 CH IR stretch is obscured by CHCl3 solvent.) All evidence points to an aromatic ester. NMR integration shows 4 H’s on ring. Conclusion: it must be disubstituted. H A’s are identical by symmetry. HB’s are also identical. Although both HA & HB are aromatic H’s, they are in slightly different environments (one is closer to X, the other to Y), so two different δ’s are observed. HA & HB couple with each other to give doublets (one neighbor each). X HA HA HB HB Y But what are X & Y? We know it must have a conjugated ester: aromatic-CO2R. What is R? We have used up 7 C’s, leaving 2 C’s, so guess R = Et. Et shows characteristic triplet of area 3 & quartet of area 2 in 1H NMR. The -HC2- has 3 neighbors (thus quartet), and is downfield because it is on oxygen. What is left? From MF only NH2 remains. This is consistent with IR at ν = 3400 and 3500 cm-1. Correct answer: O O A C D E NH2 B H’s directly N (or O) are typically broad singlets. In this case it overlaps with the quartet. 8. M F : IR: 13 C4H 8O2 (Would its molecular mass be even or odd?) No broad peaks above 3000 cm-1, ∴ no -OH, -NH. ν = 1740 cm-1, ∴ C=O present. In the range of aldehyde or ester; too high for most ketones No sharp peaks above 3000 cm-1, ∴ no sp2 CH bonds (C=C unlikely) C NMR: Four unique carbons are seen (ignore peak from carbon in solvent, CDCl3). One of them is characteristic of C=O (δ 171 ppm) and one is adjacent to heteroatom (δ 60 ppm). 1 H NMR: Three types of hydrogen (three unique environments) δ (ppm) Area Multiplicity A 1.3 3 t B 2.1 3 s C 4.1 2 q What has 2 O’s? (a) diol (b) di-ether (c) diketone (d) di-aldehyde (e) carboxylic acid (f) ester How many degrees of unsaturation are there? Fully saturated would be Cn H 2n+2, or C4H 10, so it is missing 2H’s, which is 1 degree of unsaturation. Only 1 ring or 1 double bond. That rules out diketone and dialdehyde. Absence of OH peak rules out diol and carboxylic acid. Diol and di-ether are ruled out because they have no C=O. Only ester is left. Its C=O is the one degree of saturation. The IR C=O is typical of a generic, unconjugated ester. There are only 2 four C esters: O O O O They would have virtually identical 13C NMR. Their 1H NMR would give identical integrals 3:3:2. They have identical splitting patterns: the typical triplet/quartet for Et and a sharp singlet for Me. They differ only in the chemical shifts. In one case the Me is attached to a double bond (expected δ ~2 ppm), in the other case it is attached to an oxygen (expected δ ~4 ppm). This Me is closer to 2 ppm, therefore it must be the first one, ethyl acetate: O O B 9. M F : IR: C A C8H 10 (Would its molecular mass be even or odd?) No broad peaks above 3000 cm-1, ∴ no -OH, -NH. No peak at ~ 1700 cm-1, ∴ no C=O present. Sharp peaks above just 3000 cm-1, ∴ sp2 CH bonds and ∴ C=C 13 C NMR: Five unique carbons signals (ignore peak from carbon in solvent, CDCl3). Four of them is characteristic of C=C (δ 120 - 160 ppm). 1 H NMR: Two types of hydrogen (two unique environments) δ (ppm) Area Multiplicity A 2.3 6 s B ~7 4 m How many degrees of unsaturation are there? Fully saturated would be Cn H 2n+2, or C8H 18, so it is missing 8H’s, which is 4 degrees of unsaturation. The MF & absence of functional groups in IR indicate that this is a hydrocarbon. Chemical shifts in both 1H & 13C indicate aromatic ring. The IR (sp2 CH) and relatively large degrees of unsaturation hint at this as well. Why does the 1H NMR show 4 H’s in the aromatic region instead of 6? Beause the aromatic ring is disubstituted. What are the two substituents? MF (C8H 10) - C6H 4 fragment = 2C’s & 6H’s. Based on the sharp singlet of area =1 in the 1H NMR, 2 Me’s are likely. Me’s must be attached to directly to the ring; the Me’s chemical shift (~2 ppm) is typical of H’s near double bonds. But what is the relative orientation of the Me’s? Ortho, meta or para? ortho meta para Why does the 13C NMR show only five peaks if the MF indicates 8 C’s? The molecule must have some symmetry. The ortho would show 4 unique signal and can thus be ruled out. The meta and para would both show 5 peaks. How can the meta and para be distinguished? By the 1H NMR coupling pattern. For the para, all 4 aromatic hydrogens are in the same environment; all 4 are identical by symmetry and identical H’s don’t couple (split). Thus it would appear as a singlet. Since it is a complicated multiplet, we conclude it must be the meta isomer. meta The 4 meta aromatic H’s are in 3 different environments: between Me’s, adjacent to Me’s and remote from Me’s, w/ coupling of singlet, doublet, and triplet (if you look closely you can pick out this coupling). (Meta substituted aromatics show unique peak in IR fingerprint region at ~900, ~800, & ~ 700 cm-1)