CH217 - NMR accompanying material
advertisement
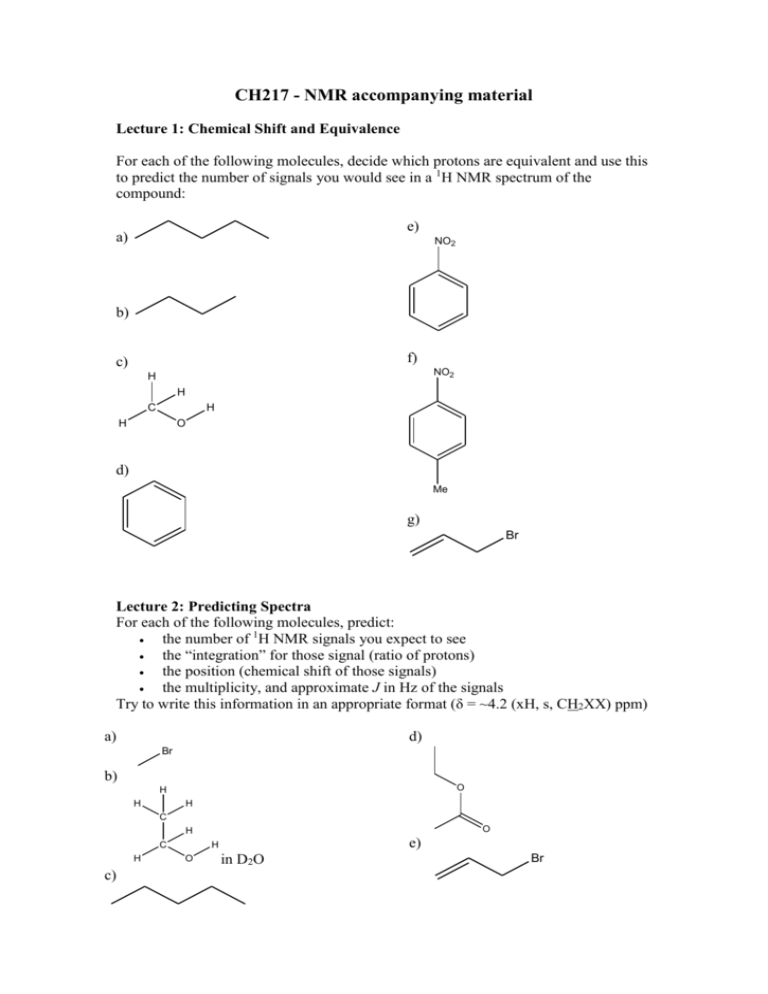
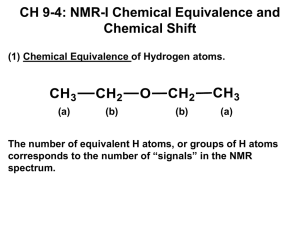
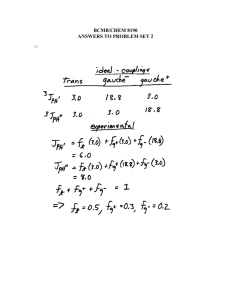
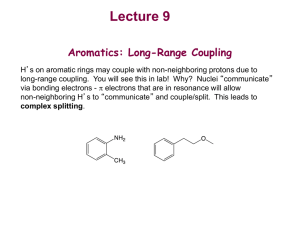
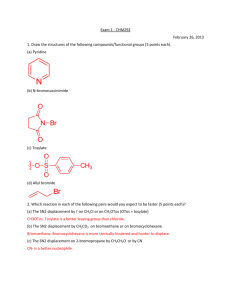
CH217 - NMR accompanying material Lecture 1: Chemical Shift and Equivalence For each of the following molecules, decide which protons are equivalent and use this to predict the number of signals you would see in a 1H NMR spectrum of the compound: e) a) NO2 b) f) c) NO2 H H C H H O d) Me g) Br Lecture 2: Predicting Spectra For each of the following molecules, predict: the number of 1H NMR signals you expect to see the “integration” for those signal (ratio of protons) the position (chemical shift of those signals) the multiplicity, and approximate J in Hz of the signals Try to write this information in an appropriate format (δ = ~4.2 (xH, s, CH2XX) ppm) a) d) Br b) O H H H C O H C H c) e) H O in D2O Br Lecture 1: Chemical Shift and Equivalence Answers a) 3 b) 2 c) 2 d) 1 e) 3 f) 3 g) 4 Lecture 2: Predicting Spectra Answers For each of the following molecules, predict: the number of 1H NMR signals you expect to see the “integration” for those signal (ratio of protons) the position (chemical shift of those signals) the multiplicity, and approximate J in Hz of the signals Try to write this information in an appropriate format: a) δ = ~3.5(1H, s, CH3Br) ppm d) δ = ~ 4.0(2H, q, J7Hz, OCH2CH3), ~2.0 (3H, s, CH3C(O)), ~ 1.2(3H, t, J7Hz, OCH2CH3) ppm Br b) δ = ~3.5(2H, q, J7Hz, CH2OH), ~1.5 (3H, t, J7Hz, CH3CH2OH), OH exchanging, ppm O H H H O C H C H H O c) δ = ~1.5(2H, quintet, J7.5Hz, CH3CH2CH2CH2CH3), ~1.2 (4H, tq, J7,7.5Hz, CH3CH2CH2CH2CH3), 1.0 (6H, t, J7 Hz, CH3CH2CH2CH2CH3) ppm e) δ = ~ 6.0 (1H, ddt, J15, 10, 7Hz, BrCH2CH=CH2), ~5.6 (1H, dd, J15, 1Hz, BrCH2CH=CH2 trans), ~5.3 (1H, dd, J10, 1Hz, BrCH2CH=CH2 cis), ~ 3.8 (2H, d, J7Hz, BrCH2CH=CH2) ppm Br