Ch 335 – Quiz 1 (take
advertisement
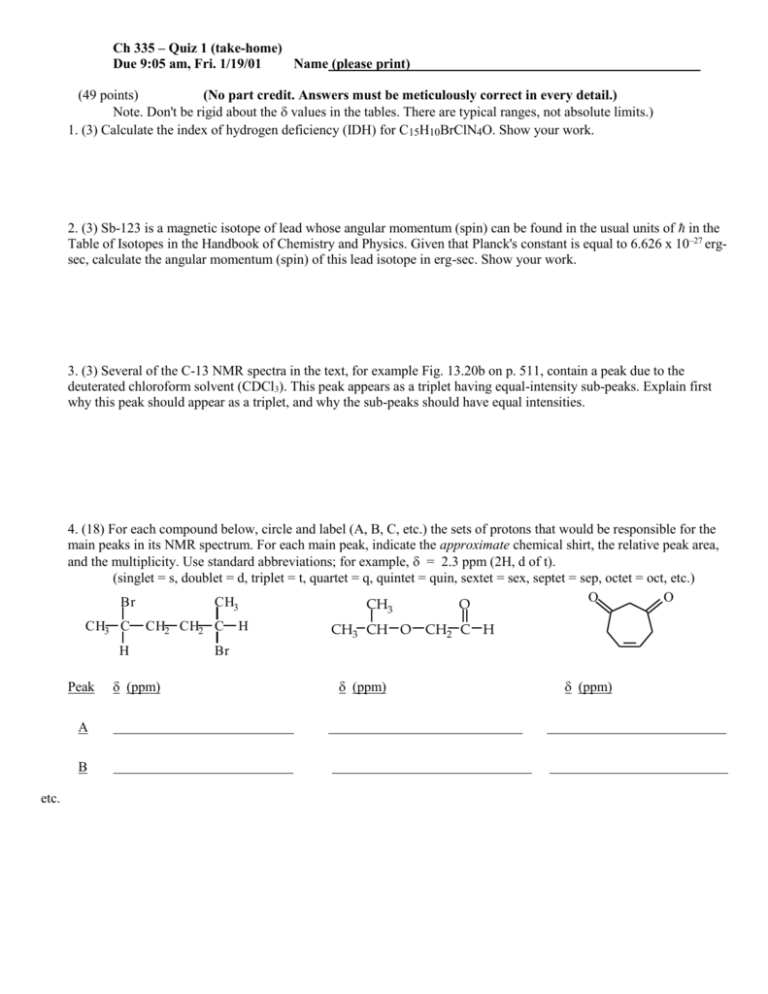
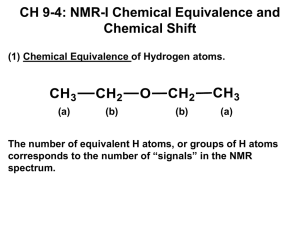
Ch 335 – Quiz 1 (take-home) Due 9:05 am, Fri. 1/19/01 Name (please print) (49 points) (No part credit. Answers must be meticulously correct in every detail.) Note. Don't be rigid about the values in the tables. There are typical ranges, not absolute limits.) 1. (3) Calculate the index of hydrogen deficiency (IDH) for C15H10BrClN4O. Show your work. 2. (3) Sb-123 is a magnetic isotope of lead whose angular momentum (spin) can be found in the usual units of in the Table of Isotopes in the Handbook of Chemistry and Physics. Given that Planck's constant is equal to 6.626 x 10–27 ergsec, calculate the angular momentum (spin) of this lead isotope in erg-sec. Show your work. 3. (3) Several of the C-13 NMR spectra in the text, for example Fig. 13.20b on p. 511, contain a peak due to the deuterated chloroform solvent (CDCl3). This peak appears as a triplet having equal-intensity sub-peaks. Explain first why this peak should appear as a triplet, and why the sub-peaks should have equal intensities. 4. (18) For each compound below, circle and label (A, B, C, etc.) the sets of protons that would be responsible for the main peaks in its NMR spectrum. For each main peak, indicate the approximate chemical shirt, the relative peak area, and the multiplicity. Use standard abbreviations; for example, = 2.3 ppm (2H, d of t). (singlet = s, doublet = d, triplet = t, quartet = q, quintet = quin, sextet = sex, septet = sep, octet = oct, etc.) O O Br CH3 CH3 O CH3 C CH2 CH2 C H Peak A B etc. (ppm) H CH3 CH O CH2 C H Br (ppm) (ppm) 5. (8) A C6H14O isomer exhibited the proton NMR spectrum tabulated below. For each resonance, indicate what part structure is suggested by the data. Propose a total structure, and justify your conclusion. (Label the proton groups in your structure, and indicate why the structure is consistent with the data.) (ppm) Part Structure A 1.1 (6H, d) B 3.6 (1H, sep) 6. (8) A C5H10O2 isomer exhibited the proton NMR spectrum tabulated below. For each resonance, indicate what part structure is suggested by the data. Propose a total structure, and justify your conclusion. (Label the proton groups in your structure, and indicate why the structure is consistent with the data.) (ppm) Part Structure A 0.9 (3H, t) B 1.6 (2H, sex) C 2.2 (2H, t) D 3.6 (3H, s) 7. (6) An isomer of C5H6O has the C-13 NMR resonances tabulated below. Propose a structure consistent with this spectrum, and justify your answer. (Label the carbons in your structure, and indicate why the structure is consistent with the data.) (ppm) A 33 B 38 C 134 D 165 E 193