How to Report NMR Spectra in a Formal Report
advertisement

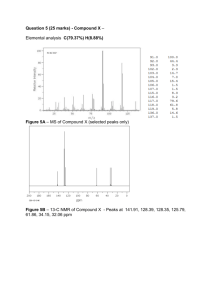
Chem117L Spring 2007 How to Report NMR Spectra in a Formal Report One of the most important elements of authoring an experimental publication is the correct reporting of analytical data. In Experiment 22, you and your lab partner took 2 NMR spectra: one of your starting material and one of your product. Both of these spectra need to be stapled to your formal report when you turn it in. In addition, you will be required to report these NMR spectra in standard written format. This spectral information must be listed in your Supplemental Information section and does not count towards the two-page limit of your report. This handout will assist you in preparing this section of your report. The 1H-NMR spectrum of the following oxoacetate derivative has been provided as an example (see attached): O O N H O Step 1. Give a little information regarding your sample. In this example, a proton NMR was taken on a 300 MHz instrument in deuterated chloroform (CDCl3). This information is reported as follows: nucleus (proton, carbon, etc.) NMR (frequency used, solvent) Filling in the data for this sample, we would write: 1 H NMR (300 Mz, CDCl3) The 1H NMR spectra in Experiment 22 were recorded on a 300 MHz instrument using DMSO-d6 as the solvent. Report accordingly. Step 2. Enter in your peak data. Immediately after the information section in Step 1, place a delta symbol (δ) to indicate that the data that follows represents peak data from the NMR. Peaks are generally reported from left to right across an NMR spectum. In the attached example, the peak furthest to the left on the spectrum is at 8.37 ppm. Round all chemical shifts to the hundredth position. See Table 1 on p2 of this handout for common splitting pattern abbreviations. Peaks are generally reported as follows: peak shift in ppm (# of H’s this peak corresponds to, splitting pattern) *Reporting environments with a splitting pattern corresponding to an odd # of peaks (e.g. a triplet): simply write down the chemical shift of the centermost peak. For example, in the attached spectrum, the 2 methines of the isopropyl groups split into a septet with the centermost (largest) peak at 3.01 ppm. This data would be reported as: 3.01 (2H, sep). Likewise, the triplet that corresponds to the CH3 of the ethyl group can be reported as: 1.46 (3H, t). For environments corresponding to more complicated splitting patterns (e.g. a triplet of doublets), write down the centermost value of the entire splitting pattern. *Reporting environments with a splitting pattern corresponding to an even # of peaks (e.g. a doublet or quartet): in this case, the ppm value that should be recorded is the chemical shift 1 Chem117L Spring 2007 directly between the innermost peaks. For example, the two innermost peaks of the quartet in the example (corresponding to the CH2) are at 4.46 and 4.43 ppm. The value exactly midway between these is 4.445 ppm. Therefore, this quartet would be recorded as: 4.44 (2H, q). Analogously, the doublet corresponding to the six isopropyl methyl groups would be written as: 1.20 (12H, d). Again, for environments corresponding to more complicated splitting patterns (e.g. a doublet of triplets), record the chemical shift of the centermost value of the entire splitting pattern. *When things get complicated: sometimes a splitting pattern is not readily discernible. For instance, in the example spectrum, the aryl hydrogens do not look like a perfect triplet or doublet—the peak heights are off. (Ignore the peak at 7.26 ppm, as this is the proton of the chloroform solvent) You may have a spectrum where all the aryl hydrogens overlap each other in a jumble. This is where you can invoke a multiplet (m) classification. Multiplets are generally reported by recording the chemical shift range that the multiplet occupies. For example, the proton at the 4-position of the aryl ring in the example would be reported as follows: 7.36-7.31 (1H, m). Step 3. Putting it all together! The 1H-NMR spectrum for this example would be reported as: 1 H NMR (300MHz, CDCl3) δ 8.36 (1H, s), 7.36-7.31 (1H, m), 7.22-7.18 (2H, m), 4.44 (2H, q), 3.01 (2H, sep), 1.46 (3H, t), 1.20 (12H, d) *One last tidbit of information: integrations do not always match up. For example, the singlet at 8.36 and the multiplet at 7.36-7.31 both correspond to 1 hydrogen, but the integration ratio is not exactly 1:1. However, they are approximately 1:1. Analyze your spectrum accordingly. Table 1. Common splitting patterns and their abbreviations. name for peak pattern equivalent neighboring number of peaks (n+1) (n) hydrogens (abbreviation) 0 1 singlet (s) 1 2 doublet (d) 2 3 triplet (t) 3 4 quartet (q) 4 5 quintet (quin) 5 6 sextet (sex) 6 7 septet (sep) 2