7H Solutions Lesson 6
advertisement

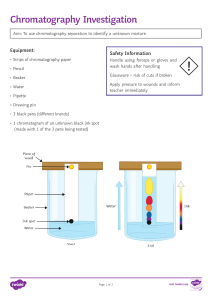
7H Solutions Lesson 6: Chromatography Learning objectives That a mixture of two or more solutes which are soluble in a particular solvent can be separated by chromatography Learning outcomes Use chromatography to separate and identify different dyes. Use particle theory to explain how chromatography works. Interpret chromatograms, explaining what the evidence shows. Resources For demonstration: “Ransom note” written with one of the test pens belonging to the suspects. For Class practical: strips of filter paper, three suspects’ pens. Lesson Outline 1. Demonstrate the separation of dyes in Quink black ink by chromatography. 2. Practical: Who wrote the note? Pupils obtain spots from three pens and produce chromatograms to see whose pen wrote the note. Hold up a “ ransom note” that was previously prepared using one of the black pens used. Prepare a chromatogram by tearing of a section of the note and get pupils to describe what they see (number of dyes in the mixture, colours of dyes in mixture). Pupils then take strips of filter paper and mark using each of the suspects’ pens in turn. Their task is to identify the kidnapper (Based on example given on P32-33 of Eureka book1). They could perhaps prepare a report to be used in court to convict the perpetrator of this heinous crime. 3. Explanation: Water creeps up the paper as soon as it is placed in the beaker. The most soluble dye is carried faster and travels further up the paper with the water. You could perhaps model this by having pupils acting our roles of water and solute particles – the levels of attraction determining how far the solute particles will go with the water particles. 4. Notes: based on explanation (missing words?). Identify most soluble dye as the one that has travelled furthest with the water 5. Question. Eureka book 1 P.33 Plenary: go through answers to questions to give immediate feedback.