ABG Interpretation: Lecture Notes on Acid-Base Balance
advertisement

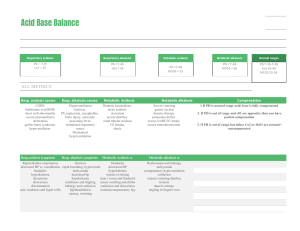
1 ABG Interpretation RSPT 1207 – Cardiopulmonary A&P Lecture Notes I. Interpretation of ABGs begins with understanding normal values first. Because of the possibility of venous blood, those values must be understood as well. II. Any values that are out of range are considered abnormal. a. pH above normal levels is alkalosis pH below normal levels is considered acidosis b. PaCO2 above normal is hypercapnia and will create acidosis PaCO2 below normal is alkalosis c. HCO3- above normal is considered alkalosis HCO3- below normal is considered acidosis d. PaO2 above normal is considered hyper-oxygenation or over-correction PaO2 below normal is considered hypoxemia III. Respiratory vs. Metabolic a. PaCO2 is a representation of the lungs ”respiratory” b. HCO3- is a representation of the renal system (kidneys) “metabolic” IV. Relationship to pH a. pH and PCO2 have an inverse relationship As CO2, pH will (acidosis) As CO2 , pH will (alkalosis) b. pH and HCO3 have a direct relationship As HCO3 , pH will (acidosis) As HCO3 , pH will (alkalosis) 2 V. ABG interpretation a. pH b. PaCO2 c. HCO3----------------------------d. PaO2 e. SaO2 Acid-Base Balance ---------------------------Oxygenation VI. Acid Base interpretation a. What is normal? What is abnormal? b. Use a method that will help you identify these things (arrows, letters, wording) c. Look at the pH first, then determine whether it is a respiratory or metabolic problem d. Examples: 7.45/43/-/25 7.25/48/-/26 7.47/43/-/28 e. Mixed acidosis or alkalosis 7.29/58/-/17 7.50/32/-/29 VII. Compensation – The body uses mechanisms to compensate for abnormalities in acid-base balance. Causes other parameters to go out of range. a. 3 categories Compensated (chronic) – when pH is inside the acceptable range. Also called fully compensated Non-compensated (acute) – when pH is outside the acceptable range. Also called uncompensated. Partial (partially) compensation – occurs when pH is out of normal range and where both CO2 and HCO3 are changing in the appropriate direction. b. pH < 7.35 = non-compensated acidosis pH > 7.45 = non-compensated alkalosis 7.27/54/-/24 7.46/32/-/22 7.26/43/-/19 7.45/41/-/28 VIII. Writing ABG Interpretation a. Traditional – Answering the following questions it is placed in this order: (4)Partially (3)Compensated (2)Respiratory (1)Acidosis 1. What is the pH (acidosis or alkalosis?) 3 2. What is the cause? (respiratory or metabolic?) 3. Is there compensation? (mixed?) 4. What type of compensation? (fully or partial) b. Acid-Base Abnormalities based on Respiratory Disorders Acute alveolar hyperventilation with hypoxemia Acute ventilatory failure with hypoxemia Chronic ventilatory failure with hypoxemia Acute alveolar hyperventilation superimposed on chronic ventilatory failure Acute ventilatory failure superimposed on chronic ventilatory failure IX. Oxygenation – Once Acid-Base balance has been established, we interpret oxygenation a. normal oxygen level 80-100 mmHg b. mild hypoxemia 60- 79 mmHg c. moderate hypoxemia 40-59 mmHg d. severe hypoxemia less than 40 mmHg If the patient's PaO2 is higher than 100 mmHg, this is interpreted as hyper-oxygenation or over correction because one may need to consider weaning the level of O2 to avoid the many hazards of O2 therapy. X. Metabolic Abnormalities a. Metabolic Acidosis Presence of other acids (ex. Lactic acid) not related to an increased PaC02 level or renal compensation. Not enough sufficient buffers being produced or when they are lost excessively Also when decreased ability to excrete acids Metabolic acidosis is present when the PaC02 is within normal range. Common causes: Lactic acidosis, ketoacidosis, renal failure, dehydration, chronic diarrhea b. Metabolic Alkalosis Presence of other bases not related to either a ↓ PaCO2 or renal compensation. Elevation of plasma HCO3 above normal or when abnormal number of H+ ions are lost. Common causes: hypokalemia, hypochloremia, gastric suction, vomiting, steroid use, Excess HCO3XI. Problems with blood gas interpretation - Bad Data 4 a. Utilize the Henderson-Hasselbach equation b. Move from 2 Standard Deviation to I Standard Deviation (tighten your range) 7.36/46/-/25 Generally when data is used to determine a range, a bell cure is created In the terms of ABGs, 95% of people have a normal pH of 7.35 – 7.45 (2SD), the middle 68% have a normal of 7.38 – 7.42 (1SD) 2 SD (95%) *pH 7.35- 7.45 *PCO2 35-45 HCO3- 21- 27 1 SD (mid 68%) pH 7.38-7.42 PCO2 38-42 *HCO322-26 * NBRC values XII. REMEMBER! When interpreting ABGs, assessment is just as important as the numbers.