File
advertisement

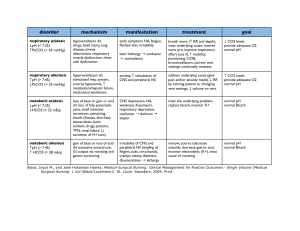
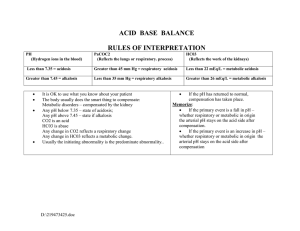
Four Major Acid-Base Imbalances and Possible Etiologies Respiratory Characteristics of Failure Imbalance Respiratory ↑ H2CO3 (carbonic acid) Acidosis level from retention of CO2. Caused by: decreased ventilation and consequent CO2 retention. “Hypoventilation” Respiratory ↓ H2CO3 (carbonic acid) Alkalosis level from excessive expiration of CO2 and H2O Caused by: increased ventilation and elimination of CO2. “Hyperventilation” Net result is ↑ pH; ↓in ionized blood calcium concentration. Associated Disease/Conditions Compensation Simple, acute respiratory acidosis results in a low pH, or acidemia. Sleep apnea, asthma, aspiration of a foreign object or ARDS. Chronic respiratory acidosis is associated with obesity hypoventilation syndrome, COPD or emphysema, certain neuromuscular diseases, and starvation cachexia. Severe, acute respiratory alkalosis results in a high pH, or alkalemia. Condition may be mediated centrally (head injury, anxiety, cerebrovascular accident, or tumors); or by peripheral stimulation (pneumonia, hypoxemia, high altitudes, pulmonary embolism, congestive heart failure, or interstitial lung disease. Also caused by aftermath of intense exercise; anxiety reaction; early sepsis. Could be produced by excessive mechanical ventilation by doctors. Kidneys compensate by ↑ bicarb resorption, thereby creating a metabolic alkalosis; which helps to ↑ the pH (takes 3-5 days). Impact to electrolytes Acidosis decreases binding of calcium to albumin and tends to increase serum ionized calcium levels. In addition, acidemia causes an extracellular shift of potassium, but respiratory acidosis rarely causes clinically significant hyperkalemia. Decrease in ionized blood calcium concentration. It could cause the symptoms of hypocalcaemia (tetany – involuntary contraction of muscles or fainting) with no fall in total serum calcium levels. Metabolic Characteristics of Failure Imbalance Metabolic ↑ H+ (decreased pH) Acidosis concentration from increased production, increased ingestion, or increased retention. OR… ↓HCO3- from extracellular fluid. (Loss of base) Associated Disease/Conditions Compensation Simple, acute metabolic acidosis results in a low blood pH, or academia. Diarrhea; uremia; ketoacidosis from uncontrolled diabetes mellitus (often resulting in rapid, deep breathing called Kussmaul respirations); toxin ingestion; starvation. A pH under 7.1 is an emergency due to risk of cardiac arrhythmias; may need IV treatment of bicarb. The lungs compensate by ↑ ventilation and CO2 elimination (rapid, deep breaths) creating a respiratory alkalosis, which helps to ↑ pH. High-fat, low-carbohydrate diet; drug use; excessive bicarb loss via the kidneys or intestinal tract; PTN without thiamin has caused death. Clinical tool: “Anion Gap” - The chloride and bicarb levels are subtracted from sodium. Four potential buffering systems: bicarb; intracellular; respiratory compensation; renal compensation. Impact to electrolytes ([Na+])-([Cl-]+[HCO3-]). Metabolic Alkalosis ↓ H+ (increased pH) concentration from increased losses. OR… ↑ HCO3- from abnormal retention of base in extracellular fluid. Elevated anion gap > 16 mmol/l can indicate particular types of metabolic acidosis. Simple, acute metabolic alkalosis results in a high blood pH (alkalemia). Diuretics use; increased ingestion of alkali; loss of acid during gastric suctioning. Loss of chloride (from villous adenoma or diuretic use); vomiting; decreased blood flow to the kidneys stimulates resorption of sodium and water, increasing bicarb resorption resulting in “contraction alkalosis”. Compensation occurs mainly in the lungs through hypoventilation. Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH. Could result in hypokalemia. Severe hypokalemia can also cause alkalosis - as K moves to extracellular fluid, H+ moves to the intracellular fluid to maintain electroneutrality resulting in intracellular acidosis - which increases H+ excretion and bicarb resorption by the kidneys.