Study Guide for Chemistry Final

Study Guide for Chemistry Final
Stoichiometry
Mole-mole & mass-mass problems, limiting reactant, % yield, % error, molecular mass of a gas
States of Matter
Kinetic energy & Theory, gas pressure (barometers, atm. Pressure, Pa, 1 atm = 760 mm Hg., etc.)
Kelvin and Celsius
Evaporation, vaporization, boiling pt., melting pt., sublimation, phase diagrams (triple point)
Gas Laws
Boyle’s law (PV = PV), Charles’s law, Combined Gas law, Ideal Gas law (PV=nRT),
Partial pressure,
Avogadro’s Hypothesis,
Diffusion vs. effusion
Electrons in Atoms
Quantum, quantum #, orbitals, electron configerations, electron dot diagrams, Lewis structures, energy levels
Amplitude, wavelength, frequency
Electromagnetic radiation, atomic emission spectrum
Planck’s Constant = h (E= hv)
Photons, photoelectric effect, ground state
Periodic Table
Periods (series), groups (families), periodic law,
Noble gases, representative elements, etc. (Know where they are and how their s, p, d, and f orbitals are filled)
Trends
Radius, ionization energy, electronegativities, density
Cations vs. anions
Activity Series
Differences between oxygen gas and ozone
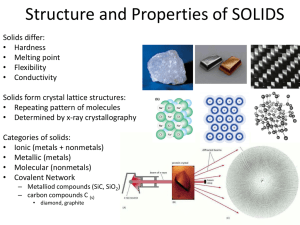
Types of Chemical Bonds
Ionic vs. covalent vs. metallic
Know how to tell if a bond is ionic, covalent, or metallic
Know when to draw single, double, and triple bonds, know about lone pairs of electrons
Polar, nonpolar, ionic bonds
Solutions
Factors that affect soln formation and dissolving
Types of solns
Liquid, solid (alloy), gas
Conc. of soln
Molarity, molality, percent by mass
Heats of soln.
Nuclear Energy
Alpha, Beta, Gamma Radiation
Nuclear Equations
Half Life Problems
Equilibrium (L3 only)
Factors affecting equilibrium
Homogenous vs Heterogeneous Equilibrium
Know the shifts