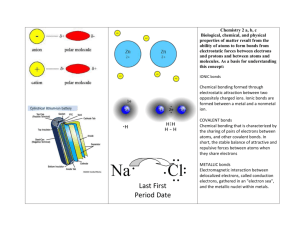
Ionic and Metallic Bonding Worksheet

IONIC AND METALLIC BONDING Name
Make the following statements correct by crossing out the incorrect underlined word(s) .
1. Anions are smaller / larger than their corresponding atoms.
2. In the formation of an ionic compound the net energy change is endothermic/exothermic.
3. When bonds are formed energy is required / released.
4. Salts are composed of 2 nonmetallic elements / a positive and a negative ion.
5. Hydroxides are salts / ionic compounds.
6. The number / arrangement of ions in a crystal determines its shape.
7. MgSO
4
is a molecule / formula unit.
8. Large attractive forces between particles in substances result in low/high melting points.
9. Ionic bonding involves a transfer / sharing of electrons.
10. The bonds in an ionic compound are strong / weak.
11. Solid / molten salt will conduct electricity.
12. NaOH / NaNO
3
is a salt.
13. Ionic compounds are solids / liquids at room temperature.
14. The energy given off when ions bond to form a solid salt is ionization / lattice energy.
15. Compounds form to achieve the lowest / highest possible energy.
16. Li
3
PO
4
/ Ba(OH)
2
is a hydroxide.
17. Metals and metal alloys are poor / good conductors of electricity.
18. Metallic bonds form between atoms with high / low ionization energies.
19. Metallic crystals are most often cubic / rhombic in shape.
20. Metallic bonds are the result of the attraction of nonmetallic anions / metallic cations
for free-floating electrons.