SUB PLANS: Calculating types of bonds using electronegativity
advertisement

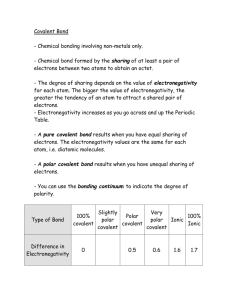
SUB PLANS: Calculating types of bonds using electronegativity chart Dr. B Use the following websites to complete this worksheet. http://www.tutorhomework.com/Chemistry_Help/electronegativity_table/electronegativity.html http://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text7/Tx71/tx71.ht ml http://www.faculty.sfasu.edu/dubenaj/che111/4_6%20Electronegativity%20an d%20Bond%20Polarity.pdf Review and Remember: To calculate the type of bond, you must first calculate the difference in electronegativity of the atoms involved in the bond. If the difference is less than .5, it is nonpolar covalent If the difference is between .5 and 1.7, it is polar covalent If the difference is over 1.7, it is ionic. 1. Why does this make sense? ___________________________________________________________________ ___________________________________________________________________ SUB PLANS: Calculating types of bonds using electronegativity chart Dr. B Worksheet – Bonding Types 1. Using only the Periodic Table and the above website, arrange the following elements in order of increasing electronegativity. Rb, Na, Li _______________ b. P, S, Al _______________ c. Fe, As, Br _______________ a. 2. Label “i” (ionic) or “c” (covalent) the bonds in the following compounds. a. CO2 __________________________ e. CaO __________ b. Na2O __________________ f. Na2CO3 (be careful) c. SF2 _____________________ ____________ d. N2O __________ 3. Calculate and group the following bonds in order of increasing ionic character. SHOW YOUR WORK BELOW. a. C-Cl NaCl Cl-Cl __________________________________________ b. N-F O-F C-F __________________________________________ c. C-F N-O Si-F ___________________________________________ d. H-Cl B-Cl S-Cl ____________________________________________ WORK: A. B. C. SUB PLANS: Calculating types of bonds using electronegativity chart Dr. B D. 4. Consider the bonds between the following pairs of elements: Mg and O, C and O, O and O, Si and O, N and O. a. b. c. Which one would be the most polar, covalent bond? ________________ Which one would be the least polar (but still polar) covalent bond? ___________ Describe the other three bonds. __________________________________________________________________ __________________________________________________________________ 5. Determine whether the molecule (or compound) is ionic, polar covalent, or nonpolar covalent. If polar covalent be sure to assign the correct partial charge ( + or - ) to each element. SHOW ALL WORK. a. N2 b. HF c. CO d. NaCl e. MgO f. AlCl3 SUB PLANS: g. CH4 h. NF3 i. CS2 j. CCl4 k. SO3 Calculating types of bonds using electronegativity chart Dr. B