CON10097.1002
advertisement
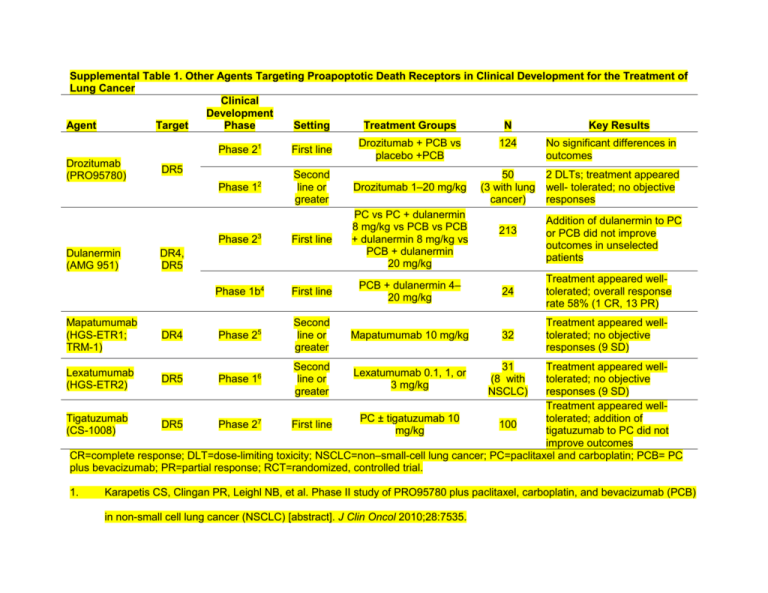
Supplemental Table 1. Other Agents Targeting Proapoptotic Death Receptors in Clinical Development for the Treatment of Lung Cancer Clinical Development Agent Target Phase Setting Treatment Groups N Key Results Drozitumab (PRO95780) 2 DLTs; treatment appeared well- tolerated; no objective responses Phase 12 Second line or greater Drozitumab 1–20 mg/kg First line PC vs PC + dulanermin 8 mg/kg vs PCB vs PCB + dulanermin 8 mg/kg vs PCB + dulanermin 20 mg/kg First line PCB + dulanermin 4– 20 mg/kg 24 Treatment appeared welltolerated; overall response rate 58% (1 CR, 13 PR) Second line or greater Mapatumumab 10 mg/kg 32 Treatment appeared welltolerated; no objective responses (9 SD) Second line or greater Lexatumumab 0.1, 1, or 3 mg/kg DR5 DR4, DR5 DR4 50 (3 with lung cancer) Drozitumab + PCB vs placebo +PCB Phase 1b Mapatumumab (HGS-ETR1; TRM-1) No significant differences in outcomes First line Phase 23 Dulanermin (AMG 951) 124 Phase 21 4 Phase 25 213 Addition of dulanermin to PC or PCB did not improve outcomes in unselected patients Treatment appeared welltolerated; no objective responses (9 SD) Treatment appeared wellTigatuzumab PC ± tigatuzumab 10 tolerated; addition of DR5 Phase 27 First line 100 (CS-1008) mg/kg tigatuzumab to PC did not improve outcomes CR=complete response; DLT=dose-limiting toxicity; NSCLC=non–small-cell lung cancer; PC=paclitaxel and carboplatin; PCB= PC plus bevacizumab; PR=partial response; RCT=randomized, controlled trial. Lexatumumab (HGS-ETR2) 1. DR5 Phase 16 31 (8 with NSCLC) Karapetis CS, Clingan PR, Leighl NB, et al. Phase II study of PRO95780 plus paclitaxel, carboplatin, and bevacizumab (PCB) in non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol 2010;28:7535. 2. Camidge DR, Herbst RS, Gordon MS, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 2010;16:1256-1263. 3. Soria JC, Mark Z, Zatloukal P, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4442-4451. 4. Soria JC, Smit E, Khayat D, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol 2010;28:1527-1533. 5. Greco FA, Bonomi P, Crawford J, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008;61:82-90. 6. Wakelee HA, Patnaik A, Sikic BI, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol 2010;21:376-381. 7. Von Pawel J, Hadler D, Fox T, et al. A randomized, double-blind, placebo-controlled phase II study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol 2012;30:7536.