Contemporary Nursing Management of EGFR Inhibitor
advertisement

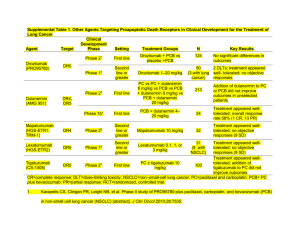
Evolving Nursing Management Strategies in the Era of Tumor Histology and Biomarker-Driven Treatment Selection for Advanced NSCLC Beth Eaby-Sandy, MSN, CRNP, OCN® Nurse Practitioner Abramson Cancer Center Disclosure of Conflicts of Interest Beth Eaby-Sandy, MSN, CRNP, OCN® discloses the following relationships: – Clovis (advisory board) – Astra Zeneca (advisory board) – Amgen (speakers’ bureau) – Merck (speakers’ bureau) – Eisai (speakers’ bureau) – Celgene (speakers’ bureau) Learning Objectives Explain the role of tumor histology and molecular biomarkers in treatment planning for advanced NSCLC Distinguish novel NSCLC therapies available for first-line therapy, maintenance therapy, and the treatment of elderly patients Apply NSCLC supportive care plans based on shared goal setting, patient health status, periodic clinical assessment, and symptom management NSCLC = non-small cell lung cancer. NSCLC: Scope of the Problem Estimated New Cancer Cases in 2015 1. Prostate: 220,800 (26%) 2. Lung/Bronchus: 115,610 (13%) 3. Colon/Rectum: 69,090 (8%) 1. Breast: 231,840 (29%) 2. Lung/Bronchus: 105,590 (13%) 3. Colon/Rectum: 63,610 (8%) ACS, 2015. NSCLC: Scope of the Problem Estimated New Cancer Deaths in 2015 1. Prostate: 27,540 (9%) 2. Lung/Bronchus: 86,380 (28%) 3. Colon/Rectum: 26,100 (8%) 1. Breast: 40,290 (15%) 2. Lung/Bronchus: 71,660 (26%) 3. Colon/Rectum: 23,600 (9%) PERSPECTIVE (Deaths) Lung/Bronchus = 158,040 (27%) Breast + Prostate + Colon/Rectum = 117,970 (20%) ACS, 2015. Lung Cancer Stages and Survival NCI, 2015. NSCLC: Breakdown by Subtype Adenocarcinoma (44%) - Most common type of NSCLC - Most common type in nonsmokers Large cell carcinoma (3%) Other (23%) Squamous (26%) – Usually more centralized – More frequently associated with significant cough and hemoptysis Houston et al, 2014. NSCLC: Molecular Biomarkers Most commonly found in adenocarcinoma, rare in squamous cell carcinoma Consider testing in squamous cell for never smokers or in mixed adenocarcinoma and squamous Three most common mutations in NSCLC: – KRAS mutation – EGFR mutation – EML4-ALK gene translocation EGFR and EML4-ALK most common in patients with never/minimal smoking history Hirsch, 2012; NCCN, 2015b. Molecular Abnormalities in NSCLC With Current Implications EGFR KRAS EML4-ALK Incidence of EML4-ALK translocation: 2-7% Estimated prevalence of EML4-ALK in lung cancer: 6,000 pts/yr US; up to 40,000 pts/yr globally Most EML4-ALK fusion events observed in lung adenocarcinoma specimens vs. squamous or small cell histologies EML4-ALK almost never coexists with EGFR, HER2, or KRAS mutations, indicating it is a distinct disease subtype Transmembrane receptor Detectable in about 80-85% of patients Level of expression varies widely Mutations in this domain (10-15% of pts) result in activation of the tyrosine kinase domain with significantly better response to erlotinib or gefitinib or afatinib Mutations: highest incidence in never smokers, adenocarcinoma, women, and patients with Asian ethnicity 25% of North American population Associated with smoking and resistance to tyrosine kinase inhibitors (TKIs) There are different types of KRAS mutations Therapy with drugs other than erlotinib should be considered first NCCN, 2015b; Langer et al, 2010. Molecular Abnormalities of Interest ROS 1 rearrangement T790M EGFR resistance mutation >60% of patients develop it after treatment Drugs in clinical trials: CO-1686, AZD9291 BRAF 4% NSCLC Most common is V600E Decreased PFS from platinum-based chemotherapy Drugs in clinical trials: dabrafenib RET 1-2% NSCLC Highly associated with young, never smokers Drugs in clinical trials: vandetanib, cabozantinib 1-2% of NSCLC Detected via FISH test Sensitive to crizotinib Usually in younger, never smokers FISH = fluorescence in situ hybridization; PFS = progression-free survival. Bergethon et al, 2012; Cardarella et al, 2013; Tsuta et al, 2014. Why Do Biomarkers Matter? PFS with standard chemotherapy regimens in NSCLC: – Pemetrexed/cisplatin: 5 months – Paclitaxel/carboplatin/bevacizumab: 6 months PFS with EGFR mutated NSCLC receiving EGFR targeted therapy: – Gefitinib: 9.5 months – Erlotinib: 9.7 months – Afatinib: 11.0 months PFS with ALK-positive NSCLC receiving ALK inhibitor: – Crizotinib: 9.7 months Camidge et al, 2012; Yang Shih, et al, 2012; Rosell et al, 2012; Fukuoka et al, 2011; Scagliotti et al, 2008; Sandler et al, 2006. Patient Factors in Treatment Planning Patient ECOG PS – PS is a predictor of survival/tolerating chemotherapy – PS 0-1 patients tolerate chemotherapy best – PS 2 patients can potentially benefit, even from doublet chemotherapy; however, toxicity must be monitored closely Comorbidities – Diabetes – Heart disease – Renal disease ECOG PS = Eastern Cooperative Oncology Group performance status. Rodriguez & Lilenbaum, 2008. Patient Factors in Treatment Planning Patient goals for treatment – Quality of life issues (eg, hair loss) – Advanced directives Demographics Social support – Involve social worker – Counseling services – Nutrition services Financial issues Supportive Care Paradigms Both ASCO and NCCN recommend integration of palliative care into oncology practice Early palliative care leads to increase in overall survival (OS) in patients with metastatic NSCLC Increased quality of life, less depressive symptoms Improved understanding of diagnosis – 1/3 patients at diagnosis thought they had curable disease – Less likely to receive chemotherapy near end of life ASCO = American Society of Clinical Oncology; NCCN = National Comprehensive Cancer Network. Temel et al, 2011; Temel et al, 2010; Smith et al, 2012; NCCN, 2015b. Case Study 1: First-Line Treatment Mrs. JF: History 68-year-old woman, presented 1 month ago with pain in her lower back Initial management with nonsteroidal antiinflamatory drugs somewhat helpful; however, the pain persisted and an xray of the lower spine was ordered X-ray did not show bone abnormality but revealed a right lung mass at the right lung base Further imaging with positron emission tomography/computed tomography (PET/CT) revealed a right lung mass, mediastinal lymphadenopathy, bone metastases in the lumbar spine, and liver metastases – X-rays are often negative Mrs. JF: Diagnostic Evaluation Treating physician referred the patient to a pulmonologist for a bronchoscopy with biopsy – Mediastinal lymph node was positive for NSCLC, adenocarcinoma histology Magnetic resonance imaging (MRI) scan of the brain negative for metastatic disease Baseline labs within normal limits Baseline PS 1 What factors important in treatment planning? Mrs. JF: Treatment Considerations Molecular analysis: Do you have enough tissue? What to test for? Which are actionable? Smoking history? Comorbidities? Weight loss, hemoptysis? Goals of care, palliative care, incurable illness: Talk to your patient about these important, yet sensitive topics! Mrs. JF: Treatment Considerations Bronchoscopy yielded core biopsy. Tissue was sent for full molecular analysis. Negative for EGFR, ALK, and ROS1 Patient has a 45-pack-year smoking history, currently trying to quit Patient has hypertension (controlled with medication), hypercholesterolemia, chronic obstructive pulmonary disease No significant weight loss, no hemoptysis Understands incurable, no advanced directive, would like to “fight” Mrs. JF: Treatment Selection Standard chemotherapy in biomarker-negative patient appropriate therapy Numerous options available for chemotherapy Platinum based chemotherapy appropriate given good PS Is hair loss an issue? Does she want to enroll in a clinical trial? NCCN, 2015b. Mrs. JF: Treatment Selection Cisplatin vs. carboplatin? In US, carboplatin is often used in frontline therapy Which drug to pair with carboplatin? Toxicity? – Pemetrexed – Paclitaxel – Docetaxel – nab-paclitaxel – Gemcitabine – Vinorelbine ECOG 1594: All Platinum Doublets Essentially Equal 1,207 patients, stage IIIB/IV (15/85%), PS 0-2 Median age 63; male/female: 64/36% R A N D O M I Z E Cisplatin Paclitaxel 75 mg/m2 D2 135 mg/m2/24h q3w Cisplatin Gemcitabine 100 mg/m2 D1 1 g/m2 D1,8,15 q4w Cisplatin Docetaxel 75 mg/m2 D1 75 mg/m2 D1 Carboplatin Paclitaxel AUC=6 mg/mL/min D1 q3w 225 mg/m2/3h D1 AUC = area under the curve. Schiller et al, 2002. Similar efficacy for all doublets q3w Importance of Histology Overall Survival Improved With Pemetrexed/Cisplatin vs. Gemcitabine/Cisplatin in First-Line Adenocarcinoma Patients Median OS (months) (95% CI) CI = confidence interval. Scagliotti et al, 2008; Scagliotti & Novello, 2003. Pemetrexed /Cisplatin Gemcitabine /Cisplatin (n=436) (n=411) 12.6 (10.7-13.6) 10.9 (10.2-11.9) E4599 Trial: Bevacizumab + PC vs. PC Alone in First-Line Nonsquamous NSCLC 1-year survival: 51% vs. 44% 2-year survival: 23% vs. 15% Sandler et al, 2006; Sandler et al, 2011. Median OS with Bevacizumab + PC was 12.3 months vs. 10.3 months for PC alone (P=0.013) PointBreak Trial: Can Regimens Be Combined? Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Paclitaxel 200 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Induction Phase q21d, 4 cycles Inclusion: − No prior systemic therapy for lung cancer − PS 0/1 − Stage IIIB-IV nonsquamous NSCLC − Stable treated brain metastasized Pemetrexed + Carboplatin + Bevacizumab R 1:1 Exclusion: − Peripheral neuropathy ≥grade 1 − Uncontrolled pleural effusions PD = progressive disease. Patel et al, 2012. Maintenance Phase q21d until PD Pemetrexed + Bevacizumab 450 Patients Each Paclitaxel + Carboplatin + Bevacizumab Bevacizumab PointBreak Trial: OS Did Not Differ Between Treatment Arms PointBreak: KM OS From Randomization (ITT) ITT = intent to treat; KM = Kaplan Meier. Patel et al, 2012. Which Regimen to Choose for First-Line Treatment? Discuss toxicity profiles of different regimens Take histology into account Give patients autonomy to decide – Do they want treatment? – If so, which regimen’s toxicity profile is right for them? – Comorbidities? Diabetes? Coronary artery disease? Renal insufficiency? Platinum-Based Chemotherapy: CINV Cisplatin is highly emetogenic; carboplatin is moderately emetogenic Following guidelines may be important in future for reimbursement Additional risk factors beyond the drug that contribute to CINV: – – – – – – – Female Younger age (<50 years) H/O low alcohol intake, <1.5 oz/d H/O motion sickness H/O morning sickness with pregnancy H/O prior CINV Anxiety CINV = chemotherapy-induced nausea and vomiting; H/O = history of. Navari, 2003; Schwartzberg, 2007; Aapro et al, 2012. Prophylaxis for High Emetic Risk Netupitant-Based Regimen Aprepitant-Based Regimen Day 1 Days 2,3,4 NCCN, 2015a. Either: - Aprepitant 125 mg PO once - Fosaprepitant 150 mg IV once 5-HT3 antagonist Dexamethasone 12 mg PO/IV once If aprepitant given Day 1: – Aprepitant 80 mg PO daily Days 2,3 – Dexamethasone 8 mg PO/IV daily Days 2,3,4 If fosaprepitant IV given Day 1: – No further aprepitant needed Days 2,3 – Dexamethasone 8 mg PO/IV once Day 2 then 8 mg PO/IV BID Days 3,4 Olanzapine-Based Regimen Netupitant 300 mg/palonesetron 0.5 mg PO once Dexamethasone 12 mg PO/IV once Dexamethasone 8 mg PO/IV daily Olanzapine 10 mg PO Palonosetron 0.25 IV once Dexamethasone 20 mg IV once Olanzapine 10 mg PO daily Prophylaxis for Moderate Emetic Risk Serotonin (5-HT3) AntagonistBased Regimen Day 1 Days 2,3 aAprepitant Netupitant-Based Regimen 5-HT3 antagonist (palonosetron 0.25 mg IV once preferred) Dexamethasone 12 mg PO/IV once ± Aprepitant 125 mg PO once or fosaprepitant 150 mg IV oncea Either: – 5-HT3 antagonist – Dexamethasone 8 mg PO/IV – If aprepitant Day 1: aprepitanta 80 mg PO daily ± dexamethasone 8 mg PO/IV – If fosaprepitant Day 1: ± Dexamethasone 8 mg PO/IV daily Olanzapine-Based Regimen Netupitant 300 mg/palonosetron 0.5 mg PO Dexamethasone 12 mg PO/IV ± Dexamethasone 8 mg PO/IV daily should be added for select patients receiving moderate emetogenic chemotherapy with additional risk factors or who have failed prior 5-HT3 and steroids. NCCN, 2015a. Olanzapine 10 mg PO once Palonosetron 0.25 mg IV once Dexamethasone 20 mg IV once Olanzapine 10 mg PO daily Case Study 1 Continued: Maintenance Therapy Mrs. JF: Post-Induction After treatment with six cycles of pemetrexed/carboplatin/bevacizumab, her tumor is stable Experienced a 20% reduction in tumor volume after two cycles, and disease has not worsened since Overall she has tolerated the regimen with minimal toxicity Her options are now: – Stop chemotherapy, best supportive care, re-scan in 2 months – Continue with maintenance chemotherapy Maintenance Therapy If no disease progression after first-line chemotherapy, continue the chemotherapy and/or targeted agent? We know that in NSCLC continuing platinum chemotherapy past four to six cycles does not improve survival, just increases toxicity However, several drugs have shown improvement in PFS in the maintenance setting NCCN, 2015b. Maintenance Pemetrexed Both studies showed improvement in OS when either switching to or continuing with maintenance pemetrexed after first-line induction platinum-based chemotherapy. Ciuleanu et al, 2009; Paz-Ares et al, 2012. PointBreak Trial: Data Did Not Favor Either Maintenance Regimen Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Paclitaxel 200 mg/m2; carboplatin AUC 6; bevacizumab 15 mg/kg Inclusion: − No prior systemic therapy for lung cancer − PS 0/1 − Stage IIIB-IV nonsquamous NSCLC − Stable treated brain metastasized Exclusion: − Peripheral neuropathy ≥grade 1 − Uncontrolled pleural effusions Patel et al, 2012. R 1:1 Induction Phase q21d, 4 cycles Maintenance Phase q21d until PD Pemetrexed + Carboplatin + Bevacizumab Pemetrexed + Bevacizumab 450 Patients Each Paclitaxel + Carboplatin + Bevacizumab Bevacizumab SATURN Trial: Erlotinib Maintenance Chemotherapy-naive, advanced NSCLC N=1,949 4 cycles of first-line platinum-based doublet Non-PD n=889 Mandatory tumor sampling SATURN included patients with the following tumor types: – Squamous cell carcinoma – Nonsquamous cell carcinoma (adenocarcinoma, large cell, other) Co-primary end points: – PFS in all patients – PFS in patients with EGFR IHC-positive tumors Secondary end points: – OS in all patients and those with EGFR IHC-positive tumors – OS and PFS in EGFR IHC-negative tumors – Safety IHC = immunohistochemistry. Cappuzzo et al, 2010. Erlotinib 150 mg/d n=438 Placebo n=451 PD PD SATURN Trial: Erlotinib Maintenance 19% reduction in risk of death OS in a broad ITT population OS rates in the SATURN ITT population at milestone Erlotinib (n=438) 1.0 Overall Survival Probability Placebo (n=451) 0.8 HR=0.81 95% CI:0.70-0.95; P=0.0088 0.6 Median 12.0 months with erlotinib vs 11.0 months with placebo 0.4 0.2 11.0 months median OS 12.0 months median OS 0 0 Cappuzzo et al, 2010. 3 6 9 12 15 18 21 Time (months) 24 27 30 33 36 Incidence and Severity of Rash Drug All Rash Incidence Cetuximab 89% (70% in FLEX trial) Grade 3/4 Incidence 12% (10% in FLEX trial) Erlotinib 75% 9% Gefitinib Rash: 43% Acne: 25% 0% (only reported ≥5%) 0% Panitumumab 89% 12% Afatinib 89% 16% Erbitux® prescribing information, 2013; Tarceva® prescribing information, 2010; Iressa® prescribing information, 2010; Vectibix® prescribing information, 2013; Yang, Shih, et al, 2012. Strategies to Prevent Dermatologic Toxicities: Pre-Emptive STEPP in metastatic colorectal cancer patients who received panitumumabcontaining regimens 95 total patients: – Significant improvement in EGFR rash and quality of life with pre-emptive doxycycline and topical hydrocortisone cream. – At 6 weeks, grade ≥2 skin toxicities were reduced by more than 50% in the pre-emptive arm STEPP = Skin Toxicity Evaluation Protocol With Panitumumab. Lacouture et al, 2010. MASCC Rash Prevention and Treatment Guidelines Preventive (Weeks 1-6, 8 of EGFR Inhibitor initiation) Recommended Not Recommended Level of Evidence Recommendation Grades Comments Doxycycline is preferred in patients with renal impairment. Minocycline is less photosensitizing. Topical • Hydrocortisone 1% cream with moisturizer and sunscreen BID • Pimecrolimus 1% cream • Tazarotene 0.05% cream • Sunscreen as single agent IIa C Systemic • Minocycline 100 mg/d Doxycyline 100 mg BID • Tetracycline 550 mg BID IIa A Topical • Alclometasone 0.05% cream • Fluocinonide 0.05% cream BID • Clindamycin 1% • Vitamin K1 Cream IVa C Fluocinonide 0.05% cream BID should not be used on the face for more than 2 weeks at a time. Systemic • Doxycycline 100 mg BID • Minocycline 100 mg/d • Isotretinoin at low doses (20-30 mg/d) • Acitretin IVa C Isotretinoin is photosensitizing and can cause xerosis. Monitor lipids and liver enzymes with retinoids. aEGFR inhibitor study. MASCC = Multinational Association of Supportive Care in Cancer. Lacouture et al, 2011. Mild Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Moderate Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Severe Rash Image courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Other Cutaneous Toxicities Alopecia/Scalp rash Paronychia Hypertrichosis Fissures Images courtesy of Beth Eaby-Sandy, MSN, CRNP, OCN® Maintenance Treatment Conclusions Again, there are options, just like in first-line chemotherapy Do patients want a break or wish to continue? Toxicity profile – Pemetrexed is chemotherapy: potential for lowering of blood counts, requires vitamin supplementation – Bevacizumab and erlotinib are targeted agents, with the potential for hypertension/cardiac toxicity, rash Cost? Should this be an issue? Insurance coverage/denials? Case Study 2: Older Adult With NSCLC Mr. PD: History Patient is an 80-year-old fit man who developed increased shortness of breath and cough during the past 6 months, though hemoptysis is what led him to the emergency department CT scan of the chest revealed a large, central lung mass as well as adrenal metastases He is a lifelong cigarette smoker, 1 pack per day CT-guided needle biopsy reveals squamous cell NSCLC Mr. PD: Diagnostic Evaluation Brain MRI scan shows a single brain metastasis 1.5 cm, for which he undergoes stereotactic brain radiation Patient presents to oncology clinic to decide about treatment options for his cancer Patient has a supportive wife and daughter; he still plays golf once a week and bridge with his friends on Wednesday nights Incidence of NSCLC in the US by Age at Diagnosis Median age at diagnosis: 70 Langer et al, 2010; SEER data, 2008-2012. Mr. PD: Treatment Considerations Chemotherapy has survival advantage over best supportive care for the fit elderly Patient would like to maintain ability to play golf and bridge and spend time with grandchildren Chemotherapy with platinum-based doublet is an option for him What can we give him that can maintain quality of life and yet give him chance for increased survival? Family wants him to pursue treatment NCCN, 2015b. IFCT-0501: Weekly PC Doublet Superior to Single-Agent Chemotherapy Overall survival (ITT) MST = median survival time. Quoix et al, 2011. nab-Paclitaxel in Elderly Patients In elderly patients, a non-significant trend toward improved PFS (8.0 vs. 6.8 months; HR=0.687; 95% CI:0.420-1.123; P=0.134) A significant improvement in OS was observed with nab-PC vs. sb-PC In patients <70 years of age, there was no difference in PFS or OS Kaplan-Meier Curve of Overall Survival in Patients ≥70 Years Probability of Survival 1.00 nab-PC (n=74) sb-PC (n=82) 0.75 19.9 months 0.50 10.4 months 0.25 HR=0.583 95% Cl:0.388-0.875 P=0.009 0.00 0 3 6 9 12 Months sb = solvent-based. Socinski et al, 2013. 15 18 21 24 Populations That Benefited Most North America Elderly (age ≥70) Squamous histology Socinski et al, 2013. Neuropathy in Elderly Patients in nab-Paclitaxel Study FACT-Taxane Results in Patients ≥70 Years 6 Peripheral Neuropathy 4.99 5 4 3 1.86 2 1 0 BL C2 Socinski et al, 2013. C3 C4 C5 C6 C7 C8 Final FACT Subscore: Mean Baseline Score or Mean Change from Baseline FACT Subscore: Mean Baseline Score or Mean Change From Baseline nab-PC 3 sb-PC Pain in Hands/Feet 2.19 2.5 2 1.5 0.70 1 0.5 0 -0.5 BL C2 C3 C4 C5 C6 C7 C8 Final Management of CIPN Complicating comorbidities, are they under control? Assessment: FACT-Taxane? Deep tendon reflexes? Vibration testing? Neurological consult for electromyography? Several studies evaluating agents such as nortriptyline, amitriptyline, gabapentin, and lamotrigine have not shown a benefit, though these agents are often used in clinical practice Duloxetine is the only agent shown to diminish CIPN in a phase III trial CIPN = chemotherapy-induced peripheral neuropathy. Eaby-Sandy, 2013. Newest Class of Agents in NSCLC: PD1 Inhibitors Well tolerated, could be used in elderly given favorable safety profile Nivolumab is only approved agent, second line after failure of chemotherapy Tumors evade immune attack by having the PD-1 ligand bind to T-cells and inactivate them against the tumor PD-1 inhibitors stop this process, thus leaving the tumor vulnerable to attack Other drugs under investigation: pembrolizumab, MPDL3280A (PD-L1), MEDI4736 (PD-L1), ipilumumab (CTLA-4), tremelumumab (CTLA-4) Cancer Research Institute, 2014. Nivolumab vs. Docetaxel: Overall Survival 100 Nivolumab n=135 Docetaxel n=137 mOS mo, (95% CI) 9.2 (7.3, 13.3) 6.0 (5.1, 7.3) # events 86 113 90 80 70 60 OS (%) 1-yr OS rate = 42% HR=0.59 (95% CI: 0.44, 0.79), P=0.00025 50 40 Nivolumab 30 20 Docetaxel 10 1-yr OS rate = 24% 0 0 3 6 9 12 15 18 21 24 Time (months) Number of Patients at Risk Nivolumab 135 113 86 69 52 31 15 7 0 Docetaxel 137 103 68 45 30 14 7 2 0 Symbols represent censored observations. Spigel et al, 2015. Treatment-Related Select AEs Nivolumab n=131 Any Grade Grade 3/4 Any Grade Grade 3/4 Endocrine, % Hypothyroidism 4 4 0 0 0 0 0 0 Gastrointestinal, % Diarrhea Colitis 8 8 1 1 0 1 20 20 0 2 2 0 Hepatic,a % ALT increased AST increased 2 2 2 0 0 0 2 1 1 1 1 1 Pulmonary, % Pneumonitis Lung infiltration Interstitial lung disease 5 5 1 0 1 1 0 0 1b 0 0 1b 0 0 0 0 Renal,c % Blood creatinine increased Tubulointerstitial nephritis 3 3 1 1 0 1 2 2 0 0 0 0 Skin,d % 9 0 9 2 Hypersensitivity/Infusion reaction, % Hypersensitivity Infusion-related reaction 1 0 1 0 0 0 2 2 1 1 1 0 Select aNo Docetaxel n=129 AEs: AEs with potential immunologic etiology that require frequent monitoring/intervention cases of increased bilirubin occurred in the nivolumab arm. bGrade 5 event. cNo cases of renal failure were reported in the nivolumab arm. dIncludes rash, pruritus, erythema, maculopapular rash, skin exfoliation, urticaria, and hand-foot syndrome. Spigel et al, 2015. Key Takeaways Treatment strategies for advanced NSCLC continue to evolve: maintenance, more aggressive treatment for elderly patients Toxicity profiles can vary significantly depending on selected agent(s) Oncology nurses play an important role in monitoring for and managing toxicities, as well as providing patient education References Aapro M, Molassiotis A, Dicato M, et al (2012). The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol, 23(8):1986-1992. American Cancer Society (2015). Cancer facts and figures 2015. Available at: www.cancer.org Avastin® (bevacizumab) prescribing information (2013). San Francisco, CA: Genentech USA, Inc. Bergethon K, Shaw AT, Ou SI, et al (2012). ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol, 30(8):863870. Bristol-Myers Squibb (2014). Study of BMS-936558 (nivolumab) compared to docetaxel in previously treated advanced or metastatic squamous cell non-small cell lung cancer (NSCLC) (CheckMate 017). Available at: http://www.clinicaltrials.gov. NLM Identifier: NCT01642004. Camidge DR, Bang YJ, Kwak EL, et al (2012). Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol, 13(10):1011-1019. Cancer Research Institute (2014). Cancer Immunotherapy: lung cancer. Available at: http://www.cancerresearch.org/cancerimmunotherapy/impacting-all-cancers/lung-cancer Cappuzzo F, Ciuleanu T, Stelmakh L, et al (2010). Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicenter, randomized, placebo-controlled phase 3 study. Lancet, 11(6):521-529. Cardarella S, Ogino A, Nishino M, et al (2013). Clinical, pathological and biological features associated with BRAF mutations in non-small cell lung cancer. Ciuleanu T, Brodowicz T, Zielinski C, et al (2009). Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet, 374(9699):1432-1440. Eaby-Sandy B, Ko A, Renschler et al (2013). Efficacy and toxicity profile of nab-paclitaxel in patients with advanced non-small cell lung cancer (NSCLC): nursing implications and management strategies. Presented at: Oncology Nursing Society 38th Congress; Washington, DC. Erbitux® (cetuximab) prescribing information (2013). New York, NY: ImClone Systems, Inc and Princeton, NJ: Bristol-Myers Squibb Co. References Fukuoka M, Wu YL, Thongprasert S, et al (2011). Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol, 29(21):2866-2874. Hirsch FR (2012). Recent advances in biomarker research in lung cancer with special reference to new targeted therapies. Presented at: 13th International Lung Cancer Congress; July 19-22, 2012; Huntington Beach, CA. Houston KA, Henley SJ, Li J, et al (2014). Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer, 86(1):22-28. Iressa® (gefitinib) prescribing information (2010). Wilmington, DE: AstraZeneca Pharmaceuticals LP. Kim Y, Oh I, Kim K, et al (2014). A randomized phase III study of docetaxel plus cisplatin versus pemetrexed plus cisplatin in first line nonsquamous non-small cell lung cancer (NSQ-NSCLC). Ann Oncol (2014 ESMO Meeting Abstracts), 25(suppl 4). Abstract LBA41_PR. Lacouture ME, Mitchell EP, Piperdi B, et al (2010). Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol, 28(8):1351-1357. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al (2011). Clinical practice guidelines for the prevention and treatment of EGFR inhibitorassociated dermatologic toxicities. Support Care Cancer, 19(8):1079-1095. Langer CJ, Besse B, Gualberto A, et al (2010). The evolving role of histology in the management of advanced non-small cell lung cancer. J Clin Oncol, 28(36):5311-5320. Lynch TJ Jr, Kim ES, Eaby B, et al (2007). Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist, 12:610-621. National Cancer Institute (2015). SEER stat fact sheets: lung and bronchus cancer. Available at: http://seer.cancer.gov/statfacts/html/lungb.html National Comprehensive Cancer Network (2015) NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer. Available at: http://www.nccn.org National Comprehensive Cancer Network (2015). NCCN Clinical Practice Guidelines in Oncology: antiemesis. Available at: http;//www.nccn.org References National Comprehensive Cancer Network (2015). NCCN Clinical Practice Guidelines in Oncology: myeloid growth factors. Available at: http://www.nccn.org Navari RM (2003). Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting—two new agents. J Support Oncol, 1(2):89103. Patel JD, Socinski MA, Garon EB, et al (2012). A randomized, open-label, phase III, superiority study of pemetrexed (Pem) + carboplatin (Cb) + bevacizumab (Bev) followed by maintenance Pem + Bev versus paclitaxel (Pac) Cb + Bev followed by maintenance Bev in patients with sage IIIB or IV non-squamous non-small cell lung cancer (NS-NSCLC). Presented at: 2012 Chicago Multidisciplinary Symposium in Thoracic Oncology. Available at: http://www.thoracicsymposium.org/MeetingProgram/documents/PLPatel.pdf Paz-Ares L, de Marinis F, Dediu M, et al (2012). Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol, 13(3):247-255. Quoix E, Zalcman G, Oster JP, et al (2011). Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet, 378(9796):1079-1088. Rodriguez E & Lilenbaum RC (2008). New treatment strategies in patients with advanced non-small-cell lung cancer and performance status 2. Clin Lung Cancer, 9(6):326-330. Rosell R, Carcereny E, Gervais R, et al (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncology, 13(3):239-246. Sandler A, Gray R, Perry MC, et al (2006). Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med, 355(24):2542-2550. Sandler A, Graham C, Baggstrom M, et al (2011). An open-label, multicenter, three-stage, phase II study of S-1 in combination with cisplatin as first-line therapy for patients with advanced non-small cell lung cancer. J Thoracic Oncol, 6(8):1400-1406. Scagliotti GV, Parikh P, von Pawel J, et al (2008). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage nonsmall-cell lung cancer. J Clin Oncol, 20;26(21):3543-3551. References Scagliotti GV & Novello S (2003). Pemetrexed and its emerging role in the treatment of thoracic malignancies. Expert Opin Investig Drugs, 12(5):853-863. Schiller JH, Harrington D, Belani CP, et al (2002). Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med, 346(2):92-98. Schwartzberg JS (2007). Chemotherapy-induced nausea and vomiting: clinician and patient perspectives. J Support Oncol, 5(2 suppl 1):5-12. Smith TJ, Temin S, Alesi ER, et al (2012). American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol, 30(8):880-887. Socinski MA, Langer CJ, Okamoto I, et al (2013). Safety and efficacy of weekly nab ®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol, 24(2):314-321. Temel JS, Greer JA, Muzikansky A, et al (2010). Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med, 363(8):733-742. Temel JS, Greer JA, Admane S, et al (2011). Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-smallcell lung cancer: results of a randomized study of early palliative care. J Clin Oncol, 29(17):2319-2326. Tsuta K, Kohno T, Yoshida A, et al (2014). RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer, 110(6):1571-1578. Tarceva® (erlotinib) prescribing information (2010). Melville, NY: OSI Pharmaceuticals, Inc. Vectibix® (panitumumab) prescribing information (2013). Thousand Oaks, CA: Amgen, Inc. Yang JC, Shih JY, Su WC, et al (2012). Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUXLung 2): a phase 2 trial. Lancet Oncol, 13(5):539-548.